当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Syntheses of Prenylated Isoflavones from Erythrina sacleuxii and Their Antibacterial Activity: 5-Deoxy-3′-prenylbiochanin A and Erysubin F
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.jnatprod.0c00932 George Kwesiga 1 , Alexandra Kelling 1 , Sebastian Kersting 2 , Eric Sperlich 1 , Markus von Nickisch-Rosenegk 2 , Bernd Schmidt 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.jnatprod.0c00932 George Kwesiga 1 , Alexandra Kelling 1 , Sebastian Kersting 2 , Eric Sperlich 1 , Markus von Nickisch-Rosenegk 2 , Bernd Schmidt 1
Affiliation
|
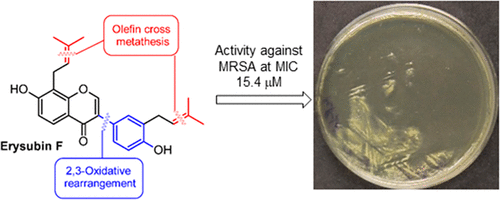
The prenylated isoflavones 5-deoxyprenylbiochanin A (7-hydroxy-4′-methoxy-3′-prenylisoflavone) and erysubin F (7,4′-dihydroxy-8,3′-diprenylisoflavone) were synthesized for the first time, starting from mono- or di-O-allylated chalcones, and the structure of 5-deoxy-3′-prenylbiochanin A was corroborated by single-crystal X-ray diffraction analysis. Flavanones are key intermediates in the synthesis. Their reaction with hypervalent iodine reagents affords isoflavones via a 2,3-oxidative rearrangement and the corresponding flavone isomers via 2,3-dehydrogenation. This enabled a synthesis of 7,4′-dihydroxy-8,3′-diprenylflavone, a non-natural regioisomer of erysubin F. Erysubin F (8), 7,4′-dihydroxy-8,3′-diprenylflavone (27), and 5-deoxy-3′-prenylbiochanin A (7) were tested against three bacterial strains and one fungal pathogen. All three compounds are inactive against Salmonella enterica subsp. enterica (NCTC 13349), Escherichia coli (ATCC 25922), and Candida albicans (ATCC 90028), with MIC values greater than 80.0 μM. The diprenylated natural product erysubin F (8) and its flavone isomer 7,4′-dihydroxy-8,3′-diprenylflavone (27) show in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA, ATCC 43300) at MIC values of 15.4 and 20.5 μM, respectively. In contrast, the monoprenylated 5-deoxy-3′-prenylbiochanin A (7) is inactive against this MRSA strain.
中文翻译:

Erythrina sacleuxii 异黄酮的全合成及其抗菌活性:5-Deoxy-3'-prenylbiochanin A 和 Erysubin F
首次合成了异黄酮 5-deoxyprenylbiochanin A (7-hydroxy-4'-methoxy-3'-prenylisoflavone) 和 erysubin F (7,4'-dihydroxy-8,3'-diprenylisoflavone)。 - 或二-O-烯丙基化查耳酮,5-脱氧-3'-异戊二烯生物链素A的结构通过单晶X射线衍射分析得到证实。黄烷酮是合成中的关键中间体。它们与高价碘试剂反应通过 2,3-氧化重排得到异黄酮,通过 2,3-脱氢得到相应的黄酮异构体。这使得合成 7,4'-二羟基-8,3'-二异戊二烯黄酮,一种赤霉素 F 的非天然区域异构体。 Erysubin F ( 8 ), 7,4'-二羟基-8,3'-二异戊二烯黄酮 ( 27 ) , 和 5-deoxy-3'-prenylbiochanin A (7 ) 针对三种细菌菌株和一种真菌病原体进行了测试。所有三种化合物对沙门氏菌亚种均无活性。肠(NCTC 13349)、大肠杆菌(ATCC 25922) 和白色念珠菌(ATCC 90028),MIC 值大于 80.0 μM。在 MIC 值为 15.4 的情况下,二异戊二烯化天然产物赤藓糖苷 F ( 8 ) 及其黄酮异构体 7,4'-二羟基-8,3'-二异戊二烯黄酮 ( 27 ) 显示出对耐甲氧西林金黄色葡萄球菌(MRSA, ATCC 43300 ) 的体外活性和 20.5 μM,分别。相比之下,单异戊二烯化 5-脱氧-3'-异戊二烯生物链菌素 A ( 7 ) 对该 MRSA 菌株无活性。
更新日期:2020-11-25
中文翻译:

Erythrina sacleuxii 异黄酮的全合成及其抗菌活性:5-Deoxy-3'-prenylbiochanin A 和 Erysubin F
首次合成了异黄酮 5-deoxyprenylbiochanin A (7-hydroxy-4'-methoxy-3'-prenylisoflavone) 和 erysubin F (7,4'-dihydroxy-8,3'-diprenylisoflavone)。 - 或二-O-烯丙基化查耳酮,5-脱氧-3'-异戊二烯生物链素A的结构通过单晶X射线衍射分析得到证实。黄烷酮是合成中的关键中间体。它们与高价碘试剂反应通过 2,3-氧化重排得到异黄酮,通过 2,3-脱氢得到相应的黄酮异构体。这使得合成 7,4'-二羟基-8,3'-二异戊二烯黄酮,一种赤霉素 F 的非天然区域异构体。 Erysubin F ( 8 ), 7,4'-二羟基-8,3'-二异戊二烯黄酮 ( 27 ) , 和 5-deoxy-3'-prenylbiochanin A (7 ) 针对三种细菌菌株和一种真菌病原体进行了测试。所有三种化合物对沙门氏菌亚种均无活性。肠(NCTC 13349)、大肠杆菌(ATCC 25922) 和白色念珠菌(ATCC 90028),MIC 值大于 80.0 μM。在 MIC 值为 15.4 的情况下,二异戊二烯化天然产物赤藓糖苷 F ( 8 ) 及其黄酮异构体 7,4'-二羟基-8,3'-二异戊二烯黄酮 ( 27 ) 显示出对耐甲氧西林金黄色葡萄球菌(MRSA, ATCC 43300 ) 的体外活性和 20.5 μM,分别。相比之下,单异戊二烯化 5-脱氧-3'-异戊二烯生物链菌素 A ( 7 ) 对该 MRSA 菌株无活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号