当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isobaric Vapor–Liquid Equilibria for Binary Mixtures of Gamma-Valerolactone + Toluene
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.jced.0c00791 Munaf Al-Lami 1, 2 , Dávid Havasi 1 , Katalin Koczka 1 , László T. Mika 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.jced.0c00791 Munaf Al-Lami 1, 2 , Dávid Havasi 1 , Katalin Koczka 1 , László T. Mika 1
Affiliation
|
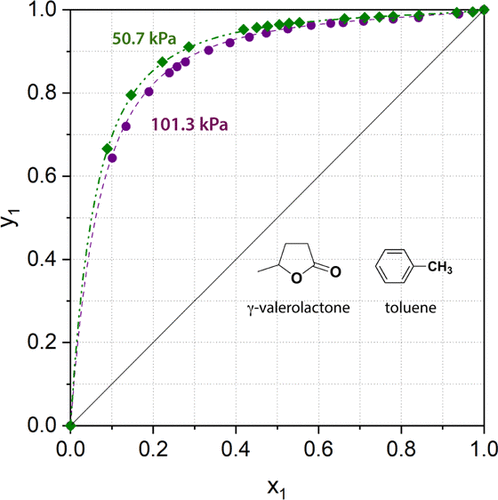
Because of the outstanding physical and chemical properties of biomass-originated γ-valerolactone (GVL), it has been characterized as a promising alternative platform molecule, which can be utilized as an oxygenate in fuels and green solvents. Herein, the isobaric vapor–liquid equilibrium (VLE) of GVL and toluene, which was selected as a representative of aromatic hydrocarbons, was investigated at p = 101.3 and 50.7 kPa. The experimental data were correlated with Wilson, NRTL, and UNIQUAC activity coefficient models. The temperature-dependent vapor pressure of toluene was additionally determined and correlated with the three-parameter Antoine and Clark–Glew equations. All the applied models were found suitable for representing the VLE data. It was found that the toluene (1)–GVL (2) mixture shows a positive deviation from Raoult’s ideality as well as from the UNIFAC prediction at both pressures.
中文翻译:

γ-戊内酯+甲苯的二元混合物的等压蒸气-液体平衡
由于生物质来源的γ-戊内酯(GVL)杰出的物理和化学性质,它已被表征为一种有前途的替代平台分子,可以用作燃料和绿色溶剂中的含氧化合物。在此,等压汽-液平衡GVL和甲苯(VLE),将其选定为芳族烃的代表,在研究了p= 101.3和50.7 kPa。实验数据与Wilson,NRTL和UNIQUAC活动系数模型相关。还确定了与温度有关的甲苯蒸气压,并将其与三参数Antoine和Clark-Glew方程相关联。发现所有应用的模型都适合表示VLE数据。研究发现,在两种压力下,甲苯(1)–GVL(2)混合物均与Raoult理想值和UNIFAC预测值存在正偏差。
更新日期:2021-01-14
中文翻译:

γ-戊内酯+甲苯的二元混合物的等压蒸气-液体平衡
由于生物质来源的γ-戊内酯(GVL)杰出的物理和化学性质,它已被表征为一种有前途的替代平台分子,可以用作燃料和绿色溶剂中的含氧化合物。在此,等压汽-液平衡GVL和甲苯(VLE),将其选定为芳族烃的代表,在研究了p= 101.3和50.7 kPa。实验数据与Wilson,NRTL和UNIQUAC活动系数模型相关。还确定了与温度有关的甲苯蒸气压,并将其与三参数Antoine和Clark-Glew方程相关联。发现所有应用的模型都适合表示VLE数据。研究发现,在两种压力下,甲苯(1)–GVL(2)混合物均与Raoult理想值和UNIFAC预测值存在正偏差。



























 京公网安备 11010802027423号
京公网安备 11010802027423号