当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hybrid Absorption–Crystallization Strategies for the Direct Air Capture of CO2 Using Phase-Changing Guanidium Bases: Insights from in Operando X-ray Scattering and Infrared Spectroscopy Measurements
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.iecr.0c03863 Meishen Liu 1 , Radu Custelcean 2 , Soenke Seifert 3 , Ivan Kuzmenko 3 , Greeshma Gadikota 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-11-11 , DOI: 10.1021/acs.iecr.0c03863 Meishen Liu 1 , Radu Custelcean 2 , Soenke Seifert 3 , Ivan Kuzmenko 3 , Greeshma Gadikota 1
Affiliation
|
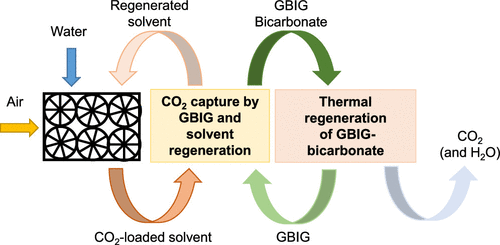
Efforts to limit rising concentrations of CO2 have motivated the development of negative emission technologies. Direct air capture (DAC) of CO2 is one of the negative emissions technologies that has been proposed for the direct removal of CO2 from the atmosphere. Phase-changing bis(iminonoguanidine) (BIG) sorbents have been developed for the direct air capture of CO2. These phase changing sorbents, specifically glyoxal-bis(iminoguanidine) (GBIG), involve (1) CO2 absorption with aqueous amino acid salts, such as K- or Na-glycinate to yield bicarbonate-rich solutions, (2) crystallization of the bicarbonate anions with a BIG solid, which regenerates the amino acid, and (3) solid-state CO2 release from the carbonate crystals and BIG regeneration. Despite the promising potential of these materials, their structural evolution during the thermal regeneration of the BIG solids, chemical regeneration of the sodium or potassium glycinate solvents, and the crystallization behavior of CO2-loaded BIG bicarbonate remain to be evaluated and understood in detail. The aim of this study is to probe these knowledge gaps. In situ wide-angle X-ray Scattering (WAXS) results show that CO2 and water molecules in GBIG bicarbonate are simultaneously released in a single step during the thermal regeneration of the sorbent at 97 – 134 °C. In situ ATR-FTIR measurements showed that sodium glycinate and GBIG bicarbonate are simultaneously generated when GBIG, glycine, and sodium bicarbonate are reacted. The crystallization of GBIG bicarbonate from GBIG and CO2-loaded monoethanolamine (MEA) occurs rapidly in the first 10 min of the reaction, as determined using in situ GI-SAXS measurements. The insights from these studies are essential for the scalable implementation of CO2 capture technologies using these phase-changing sorbents.
中文翻译:

利用相变胍碱直接捕获空气中的CO 2的混合吸收-结晶策略:Operando X射线散射和红外光谱测量的见解
限制CO 2浓度上升的努力已经推动了负排放技术的发展。CO 2的直接空气捕获(DAC)是已提出的用于从大气中直接去除CO 2的负排放技术之一。相变双(亚氨基胍)(BIG)吸附剂已被开发用于直接空气捕集CO 2。这些相变吸附剂,特别是乙二醛-双(亚氨基胍)(GBIG),涉及(1)用氨基酸水溶液(例如K-甘氨酸或Na-甘氨酸盐)吸收CO 2以产生富含碳酸氢盐的溶液,(2)含BIG固体的碳酸氢根阴离子可再生氨基酸,以及(3)固态CO 2从碳酸盐晶体中释放出来并产生大的再生。尽管这些材料具有潜在的潜力,但是它们在BIG固体的热再生,甘氨酸钠或甘氨酸盐溶剂的化学再生以及装载CO 2的BIG碳酸氢盐的结晶行为期间的结构演变仍待评估和详细了解。这项研究的目的是探究这些知识差距。原位广角X射线散射(WAXS)结果表明,CO 2在97 – 134°C的吸附剂热再生过程中,GBIG碳酸氢盐中的水和分子同时一步释放。原位ATR-FTIR测量表明,当GBIG,甘氨酸和碳酸氢钠反应时,会同时生成甘氨酸钠和GBIG碳酸氢盐。如使用原位GI-SAXS测量所确定的,在反应的前10分钟内,由GBIG和负载有CO 2的单乙醇胺(MEA)结晶出GBIG碳酸氢盐。这些研究的见解对于使用这些相变吸附剂的可扩展实施CO 2捕集技术至关重要。
更新日期:2020-11-25
中文翻译:

利用相变胍碱直接捕获空气中的CO 2的混合吸收-结晶策略:Operando X射线散射和红外光谱测量的见解
限制CO 2浓度上升的努力已经推动了负排放技术的发展。CO 2的直接空气捕获(DAC)是已提出的用于从大气中直接去除CO 2的负排放技术之一。相变双(亚氨基胍)(BIG)吸附剂已被开发用于直接空气捕集CO 2。这些相变吸附剂,特别是乙二醛-双(亚氨基胍)(GBIG),涉及(1)用氨基酸水溶液(例如K-甘氨酸或Na-甘氨酸盐)吸收CO 2以产生富含碳酸氢盐的溶液,(2)含BIG固体的碳酸氢根阴离子可再生氨基酸,以及(3)固态CO 2从碳酸盐晶体中释放出来并产生大的再生。尽管这些材料具有潜在的潜力,但是它们在BIG固体的热再生,甘氨酸钠或甘氨酸盐溶剂的化学再生以及装载CO 2的BIG碳酸氢盐的结晶行为期间的结构演变仍待评估和详细了解。这项研究的目的是探究这些知识差距。原位广角X射线散射(WAXS)结果表明,CO 2在97 – 134°C的吸附剂热再生过程中,GBIG碳酸氢盐中的水和分子同时一步释放。原位ATR-FTIR测量表明,当GBIG,甘氨酸和碳酸氢钠反应时,会同时生成甘氨酸钠和GBIG碳酸氢盐。如使用原位GI-SAXS测量所确定的,在反应的前10分钟内,由GBIG和负载有CO 2的单乙醇胺(MEA)结晶出GBIG碳酸氢盐。这些研究的见解对于使用这些相变吸附剂的可扩展实施CO 2捕集技术至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号