当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanochromic Luminescence of N,N′-Dioxide-4,4′-bipyridine Bismuth Coordination Polymers
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.cgd.0c00872 Oksana Toma 1 , Nicolas Mercier 1 , Magali Allain 1 , Francesco Meinardi 2 , Alessandra Forni 3 , Chiara Botta 4
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.cgd.0c00872 Oksana Toma 1 , Nicolas Mercier 1 , Magali Allain 1 , Francesco Meinardi 2 , Alessandra Forni 3 , Chiara Botta 4
Affiliation
|
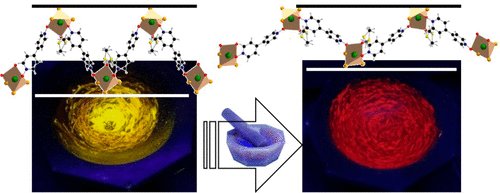
The association of Bi3+ ions and N,N′-dioxide-4,4′-bipyridine (bp4do) ligands has led to three related compounds [Bi2Br6(bp4do)(dmso)4] (1), [BiBr3(bp4do)(dmso)] (2), and [Bi2Br6(bp4do)3] (3). 2 can be described from 1, and 3 from 2, by the substitution of two dmso molecules by one bp4do molecule, the octahedral geometry of bismuth ions being kept as BiBr3O(bp4do)O2(dmso) in 1, BiBr3O2(bp4do)O(dmso) in 2, and BiBr3O3(bp4do) in 3. In the absorption spectra of 1–3, as well as Bi/bp4do complexes in solution, a metal-to-ligand charge transfer band is observed as a result of the formation of Bi–O(N-oxide) bonds. All 1–3 materials exhibit luminescence properties of phosphorescence type with quantum yields up to 54% for 1. In contrast with 1 (isolated complexes in the solid state), 1D coordination polymer type compounds 2 and 3 exhibit mechanochromic luminescence (MCL) properties: upon grinding—a few seconds for 2—the broad emission bands of the pristine compounds centered at 575 nm (2, yellow emission) and 600 nm (3, orange emission) are shifted by 75 nm (2) or 50 nm (3) (bands centered at 650 nm), leading to a strong red emission, whereas the process is reversible by heating, fuming, or recrystallization in a few drops of solvent. These MCL properties of 2 and 3 may be related to conformational changes of polymeric chains upon grinding.
中文翻译:

N,N'-二氧化物-4,4'-联吡啶铋配位聚合物的机械变色发光。
Bi的关联3+离子和Ñ,Ñ '二氧化物-4,4'-联吡啶(bp4do)配体导致了三个相关的化合物[双2溴6(bp4do)(DMSO)4 ](1),[BIBR 3(bp4do)(dmso)](2)和[Bi 2 Br 6(bp4do)3 ](3)。2可从描述1,和3从2,通过两个替换DMSO由一个分子bp4do分子,铋离子的八面体几何形状在该状态保持BIBR 3 O(bp4do)O 2(DMSO)中1,BIBR 3 Ô 2(bp4do)O(DMSO)中2,和BiBr 3 O 3(bp4do)在3。在的吸收光谱1 - 3,以及铋/ bp4do在溶液中形成络合物时,由于形成Bi-O(N-氧化物)键,可以观察到金属到配体的电荷转移带。所有1 - 3的材料表现出与量子产率磷光型的发光特性高达54%为1。与1(固态分离的配合物)相反,一维配位聚合物类型的化合物2和3表现出机械变色发光(MCL)特性:研磨时(持续2秒钟),原始化合物的宽发射带集中在575 nm处(2,黄色发射)和600 nm(3,橙色发射)偏移75 nm(2)或50 nm(3)(以650 nm为中心的谱带),导致强烈的红色发射,而此过程可通过在几滴溶剂中加热,发烟或重结晶来逆转。这些MCL属性2和3可能与研磨时聚合物链的构象变化有关。
更新日期:2020-12-02
中文翻译:

N,N'-二氧化物-4,4'-联吡啶铋配位聚合物的机械变色发光。
Bi的关联3+离子和Ñ,Ñ '二氧化物-4,4'-联吡啶(bp4do)配体导致了三个相关的化合物[双2溴6(bp4do)(DMSO)4 ](1),[BIBR 3(bp4do)(dmso)](2)和[Bi 2 Br 6(bp4do)3 ](3)。2可从描述1,和3从2,通过两个替换DMSO由一个分子bp4do分子,铋离子的八面体几何形状在该状态保持BIBR 3 O(bp4do)O 2(DMSO)中1,BIBR 3 Ô 2(bp4do)O(DMSO)中2,和BiBr 3 O 3(bp4do)在3。在的吸收光谱1 - 3,以及铋/ bp4do在溶液中形成络合物时,由于形成Bi-O(N-氧化物)键,可以观察到金属到配体的电荷转移带。所有1 - 3的材料表现出与量子产率磷光型的发光特性高达54%为1。与1(固态分离的配合物)相反,一维配位聚合物类型的化合物2和3表现出机械变色发光(MCL)特性:研磨时(持续2秒钟),原始化合物的宽发射带集中在575 nm处(2,黄色发射)和600 nm(3,橙色发射)偏移75 nm(2)或50 nm(3)(以650 nm为中心的谱带),导致强烈的红色发射,而此过程可通过在几滴溶剂中加热,发烟或重结晶来逆转。这些MCL属性2和3可能与研磨时聚合物链的构象变化有关。

























 京公网安备 11010802027423号
京公网安备 11010802027423号