当前位置:
X-MOL 学术
›
Adv. Theory Simul.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper Dimer Anchored in g‐CN Monolayer as an Efficient Electrocatalyst for CO2 Reduction Reaction: A Computational Study
Advanced Theory and Simulations ( IF 3.3 ) Pub Date : 2020-11-09 , DOI: 10.1002/adts.202000218 Cong Wang 1 , Changyan Zhu 1 , Min Zhang 1 , Yun Geng 1 , Zhongmin Su 1, 2
Advanced Theory and Simulations ( IF 3.3 ) Pub Date : 2020-11-09 , DOI: 10.1002/adts.202000218 Cong Wang 1 , Changyan Zhu 1 , Min Zhang 1 , Yun Geng 1 , Zhongmin Su 1, 2
Affiliation
|
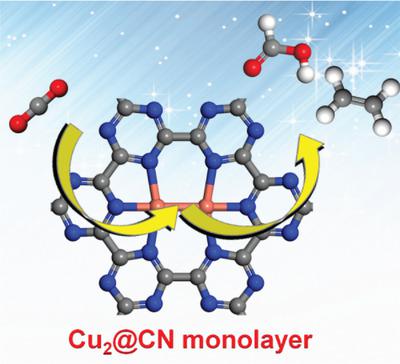
Electrocatalytic CO2 reduction (CO2RR) into value‐added energy carriers is of utmost importance due to rising emissions of CO2 and depleting energy resource. The search and design of effective, stable, and low‐cost electrocatalysts are crucial but face huge challenges. Here, the potential of copper dimer anchored in g‐CN (Cu2@CN) monolayer as electrocatalyst for CO2RR is systematically evaluated by means of density functional theory calculations. The computational results indicate that the Cu2@CN monolayer possesses superior catalytic activity for the conversion of CO2 to HCOOH and C2H4 with low limiting potential (−0.16 and −0.52 V, respectively), outperforming the corresponding single‐atom counterpart (Cu@CN). Considering the myriad of unexplored dimers anchored in/on g‐CN monolayer and their potential catalytic applications, this work provides a useful guidance for future studies.
中文翻译:

g-CN单层中锚固的铜二聚体作为一种高效的CO2还原反应电催化剂:计算研究
由于增加的CO 2排放量和消耗的能源,将电催化将CO 2还原(CO 2 RR)转化为增值能量载体至关重要。寻找,设计有效,稳定和低成本的电催化剂至关重要,但面临巨大挑战。在这里,通过密度泛函理论计算系统地评估了锚定在g-CN(Cu 2 @CN)单层中作为CO 2 RR电催化剂的铜二聚体的潜力。计算结果表明,Cu 2 @CN单层具有优异的催化活性,可将CO 2转化为HCOOH和C 2 H 4具有较低的极限电位(分别为-0.16和-0.52 V),性能优于相应的单原子对应物(Cu @ CN)。考虑到锚定在g-CN单层中/上的大量未探索的二聚体及其潜在的催化应用,这项工作为将来的研究提供了有用的指导。
更新日期:2020-12-07
中文翻译:

g-CN单层中锚固的铜二聚体作为一种高效的CO2还原反应电催化剂:计算研究
由于增加的CO 2排放量和消耗的能源,将电催化将CO 2还原(CO 2 RR)转化为增值能量载体至关重要。寻找,设计有效,稳定和低成本的电催化剂至关重要,但面临巨大挑战。在这里,通过密度泛函理论计算系统地评估了锚定在g-CN(Cu 2 @CN)单层中作为CO 2 RR电催化剂的铜二聚体的潜力。计算结果表明,Cu 2 @CN单层具有优异的催化活性,可将CO 2转化为HCOOH和C 2 H 4具有较低的极限电位(分别为-0.16和-0.52 V),性能优于相应的单原子对应物(Cu @ CN)。考虑到锚定在g-CN单层中/上的大量未探索的二聚体及其潜在的催化应用,这项工作为将来的研究提供了有用的指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号