当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phenazine Radical Cations as Efficient Homogeneous and Heterogeneous Catalysts for the Cross‐Dehydrogenative Aza‐Henry Reaction
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-11-11 , DOI: 10.1002/hlca.202000184 Felix Unglaube 1 , Paul Hünemörder 1 , Xuewen Guo 1 , Zixu Chen 2 , Dengxu Wang 2 , Esteban Mejía 1
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-11-11 , DOI: 10.1002/hlca.202000184 Felix Unglaube 1 , Paul Hünemörder 1 , Xuewen Guo 1 , Zixu Chen 2 , Dengxu Wang 2 , Esteban Mejía 1
Affiliation
|
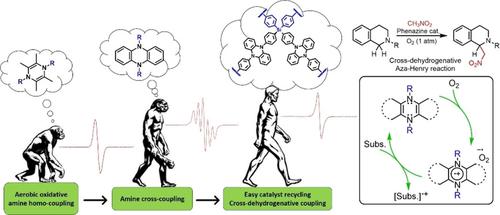
The redox activity of molecular phenazine catalysts has been previously exploited for aerobic oxidative amine homo‐ and cross‐coupling reactions. In this contribution, we have extended the reaction scope of this novel type of organocatalyst and used them in the cross‐dehydrogenative aza‐Henry coupling of isoquinolines with nitromethane under aerobic conditions. Additionally, we have designed and prepared a novel porous organic polymer by cross‐linking of tetrakis(4‐bromophenyl)silane and dihydrophenazine through Pd‐catalyzed Buchwald‐Hartwig cross‐coupling. This new type of heterogeneous catalyst, apart from being robust and easily reusable, also showed outstanding catalytic activities and improved selectivity compared to its molecular counterpart. A plausible reaction mechanism was proposed based on spectroscopic and kinetic measurements.
中文翻译:

吩嗪自由基阳离子作为交叉脱氢氮杂-亨利反应的高效均相和多相催化剂
分子吩嗪催化剂的氧化还原活性先前已用于好氧氧化胺的均相和交叉偶联反应。在这一贡献中,我们扩展了这种新型有机催化剂的反应范围,并将其用于好氧条件下异喹啉与硝基甲烷的交叉脱氢氮杂-亨利偶合。此外,我们还设计并制备了一种新型的多孔有机聚合物,该方法是通过Pd催化的Buchwald-Hartwig交联四(4-溴苯基)硅烷和二氢吩嗪交叉耦合。与它的分子对应物相比,这种新型的非均相催化剂除了坚固耐用且易于重复使用外,还表现出出色的催化活性和更高的选择性。基于光谱和动力学测量,提出了合理的反应机理。
更新日期:2020-12-11
中文翻译:

吩嗪自由基阳离子作为交叉脱氢氮杂-亨利反应的高效均相和多相催化剂
分子吩嗪催化剂的氧化还原活性先前已用于好氧氧化胺的均相和交叉偶联反应。在这一贡献中,我们扩展了这种新型有机催化剂的反应范围,并将其用于好氧条件下异喹啉与硝基甲烷的交叉脱氢氮杂-亨利偶合。此外,我们还设计并制备了一种新型的多孔有机聚合物,该方法是通过Pd催化的Buchwald-Hartwig交联四(4-溴苯基)硅烷和二氢吩嗪交叉耦合。与它的分子对应物相比,这种新型的非均相催化剂除了坚固耐用且易于重复使用外,还表现出出色的催化活性和更高的选择性。基于光谱和动力学测量,提出了合理的反应机理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号