当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CO2 Capture in Ionic Liquids Based on Amino Acid Anions With Protic Side Chains: a Computational Assessment of Kinetically Efficient Reaction Mechanisms
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-11-10 , DOI: 10.1002/open.202000275 Stefano Onofri 1 , Henry Adenusi 1 , Andrea Le Donne 1 , Enrico Bodo 1
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-11-10 , DOI: 10.1002/open.202000275 Stefano Onofri 1 , Henry Adenusi 1 , Andrea Le Donne 1 , Enrico Bodo 1
Affiliation
|
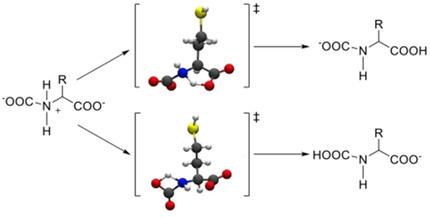
Absorption and capture of CO2 directly from sources represents one of the major tools to reduce its emission in the troposphere. One of the possibilities is to incorporate CO2 inside a liquid exploiting its propensity to react with amino groups to yield carbamic acid or carbamates. A particular class of ionic liquids, based on amino acids, appear to represent a possible efficient medium for CO2 capture because, at difference with current industrial setups, they have the appeal of a biocompatible and environmentally benign solution. We have investigated, by means of highly accurate computations, the feasibility of the reaction that incorporates CO2 in an amino acid anion with a protic side chain and ultimately transforms it into a carbamate derivative. Through an extensive exploration of the possible reaction mechanisms, we have found that different prototypes of amino acid anions present barrierless reaction mechanisms toward CO2 absorption.
中文翻译:

基于具有质子侧链的氨基酸阴离子在离子液体中捕获二氧化碳:动力学有效反应机制的计算评估
直接从源头吸收和捕获 CO 2是减少其在对流层排放的主要工具之一。一种可能性是将CO 2掺入液体中,利用其与氨基反应产生氨基甲酸或氨基甲酸酯的倾向。一类基于氨基酸的特定离子液体似乎代表了一种可能的有效的 CO 2捕获介质,因为与当前的工业设置不同,它们具有生物相容性和环境友好型解决方案的吸引力。我们已经通过高度精确的计算研究了包含 CO 2的反应的可行性在具有质子侧链的氨基酸阴离子中并最终将其转化为氨基甲酸酯衍生物。通过对可能的反应机理的广泛探索,我们发现不同的氨基酸阴离子原型对CO 2的吸收呈现出无障碍反应机理。
更新日期:2020-11-12
中文翻译:

基于具有质子侧链的氨基酸阴离子在离子液体中捕获二氧化碳:动力学有效反应机制的计算评估
直接从源头吸收和捕获 CO 2是减少其在对流层排放的主要工具之一。一种可能性是将CO 2掺入液体中,利用其与氨基反应产生氨基甲酸或氨基甲酸酯的倾向。一类基于氨基酸的特定离子液体似乎代表了一种可能的有效的 CO 2捕获介质,因为与当前的工业设置不同,它们具有生物相容性和环境友好型解决方案的吸引力。我们已经通过高度精确的计算研究了包含 CO 2的反应的可行性在具有质子侧链的氨基酸阴离子中并最终将其转化为氨基甲酸酯衍生物。通过对可能的反应机理的广泛探索,我们发现不同的氨基酸阴离子原型对CO 2的吸收呈现出无障碍反应机理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号