Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2020-11-10 , DOI: 10.1016/j.jcmgh.2020.11.003 Jana Krüger , Rüdiger Groß , Carina Conzelmann , Janis A. Müller , Lennart Koepke , Konstantin M.J. Sparrer , Tatjana Weil , Desiree Schütz , Thomas Seufferlein , Thomas F.E. Barth , Steffen Stenger , Sandra Heller , Jan Münch , Alexander Kleger
|
Background and aims
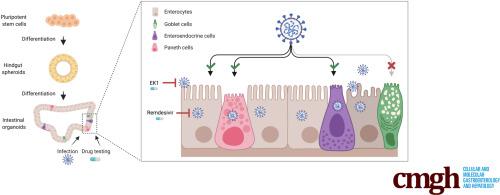
The COVID-19 pandemic has spread worldwide and poses a severe health risk. While most patients present mild symptoms, descending pneumonia can lead to severe respiratory insufficiency. Up to 50% of patients show gastrointestinal symptoms like diarrhea or nausea, intriguingly associating with prolonged symptoms and increased severity. Thus, models to understand and validate drug efficiency in the gut of COVID-19 patients are of urgent need.
Methods
Human intestinal organoids derived from pluripotent stem cells (PSC-HIOs) have led, due to their complexity in mimicking human intestinal architecture, to an unprecedented number of successful disease models including gastrointestinal infections. Here, we employed PSC-HIOs to dissect SARS-CoV-2 pathogenesis and its inhibition by remdesivir, one of the leading drugs investigated for treatment of COVID-19.
Results
Immunostaining for viral entry receptor ACE2 and SARS-CoV-2 spike protein priming protease TMPRSS2 showed broad expression in the gastrointestinal tract with highest levels in the intestine, the latter faithfully recapitulated by PSC-HIOs. Organoids could be readily infected with SARS-CoV-2 followed by viral spread across entire PSC-HIOs, subsequently leading to organoid deterioration. However, SARS-CoV-2 spared goblet cells lacking ACE2 expression. Importantly, we challenged PSC-HIOs for drug testing capacity. Specifically, remdesivir effectively inhibited SARS-CoV-2 infection dose-dependently at low micromolar concentration and rescued PSC-HIO morphology.
Conclusions
Thus, PSC-HIOs are a valuable tool to study SARS-CoV-2 infection and to identify and validate drugs especially with potential action in the gut.
中文翻译:

人多能干细胞衍生的肠类器官中SARS-CoV-2复制的药物抑制作用。
背景和目标
COVID-19大流行已蔓延至世界各地,并构成严重的健康风险。虽然大多数患者表现出轻度症状,但下降的肺炎可导致严重的呼吸功能不全。多达50%的患者表现出胃肠道症状,例如腹泻或恶心,这与长时间的症状和严重程度增加密切相关。因此,迫切需要用于理解和验证COVID-19患者肠道中药物效率的模型。
方法
源自多能干细胞(PSC-HIOs)的人类肠道类器官,由于其模仿人类肠道结构的复杂性,已导致前所未有的成功疾病模型,包括胃肠道感染。在这里,我们采用PSC-HIOs来研究SARS-CoV-2的发病机理及其对瑞姆昔韦的抑制作用,后者是研究治疗COVID-19的主要药物之一。
结果
病毒进入受体ACE2和SARS-CoV-2突突蛋白引发蛋白酶TMPRSS2的免疫染色显示在胃肠道中广泛表达,在肠道中最高水平,后者由PSC-HIO忠实地概括。类器官很容易被SARS-CoV-2感染,随后病毒传播到整个PSC-HIO,随后导致类器官退化。但是,SARS-CoV-2备用杯状细胞缺乏ACE2表达。重要的是,我们挑战了PSC-HIO的药物测试能力。具体而言,瑞姆昔韦在低微摩尔浓度下有效剂量依赖性地抑制了SARS-CoV-2感染,并挽救了PSC-HIO形态。
结论
因此,PSC-HIOs是研究SARS-CoV-2感染并鉴定和验证药物的重要工具,尤其是对肠道有潜在作用的药物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号