当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lithium separation by growth of lithium aluminum layered double hydroxides on aluminum metal substrates
Solid State Sciences ( IF 3.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.solidstatesciences.2020.106488 Yongju Lee , Ji-Hyun Cha , Duk-Young Jung
Solid State Sciences ( IF 3.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.solidstatesciences.2020.106488 Yongju Lee , Ji-Hyun Cha , Duk-Young Jung
|
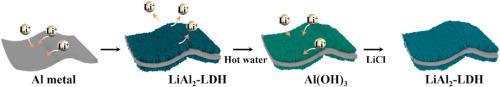
Abstract We propose a reaction scheme to selectively separate lithium ions from an aqueous urea solution by formation of lithium aluminum layered double hydroxide (LDH) on aluminum metal foils. The repeated cycles of adsorption and desorption of lithium ions are possible using the topotactic reaction of lithium salts with Al(OH)3 grown on an aluminum metal surface in aqueous solution. The urea and lithium salt concentration, pH, and reaction time are critical parameters for modulating the maximum yield of recovered lithium quantity, which is 8 μg/cm2 of aluminum metal surface. The adsorbed lithium is strongly correlated with saturation conditions of lithium and urea, dependent upon competition between the rate of crystal growth and that of nucleation of LDH. The kinetic study of sorption and surface characterization provides a possible mechanism for the lithium exchange reaction and suggests the facile methodology of a lithium recovery system.
中文翻译:

通过在铝金属基材上生长锂铝层状双氢氧化物来分离锂
摘要 我们提出了一种反应方案,通过在铝金属箔上形成锂铝层状双氢氧化物 (LDH),从尿素水溶液中选择性地分离锂离子。使用锂盐与在水溶液中铝金属表面生长的 Al(OH)3 的拓扑反应,锂离子的吸附和解吸的重复循环是可能的。尿素和锂盐的浓度、pH 值和反应时间是调节最大回收锂量(铝金属表面 8 μg/cm2)的关键参数。吸附的锂与锂和尿素的饱和条件密切相关,取决于晶体生长速率和 LDH 成核速率之间的竞争。
更新日期:2020-12-01
中文翻译:

通过在铝金属基材上生长锂铝层状双氢氧化物来分离锂
摘要 我们提出了一种反应方案,通过在铝金属箔上形成锂铝层状双氢氧化物 (LDH),从尿素水溶液中选择性地分离锂离子。使用锂盐与在水溶液中铝金属表面生长的 Al(OH)3 的拓扑反应,锂离子的吸附和解吸的重复循环是可能的。尿素和锂盐的浓度、pH 值和反应时间是调节最大回收锂量(铝金属表面 8 μg/cm2)的关键参数。吸附的锂与锂和尿素的饱和条件密切相关,取决于晶体生长速率和 LDH 成核速率之间的竞争。


























 京公网安备 11010802027423号
京公网安备 11010802027423号