当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Dynamic Structure–Function and Structure-Free Energy Relationships of the Coronavirus Main Protease with Biodynamics Theory
ACS Pharmacology & Translational Science Pub Date : 2020-11-06 , DOI: 10.1021/acsptsci.0c00089 Hongbin Wan 1 , Vibhas Aravamuthan 2 , Robert A. Pearlstein 1
ACS Pharmacology & Translational Science Pub Date : 2020-11-06 , DOI: 10.1021/acsptsci.0c00089 Hongbin Wan 1 , Vibhas Aravamuthan 2 , Robert A. Pearlstein 1
Affiliation
|
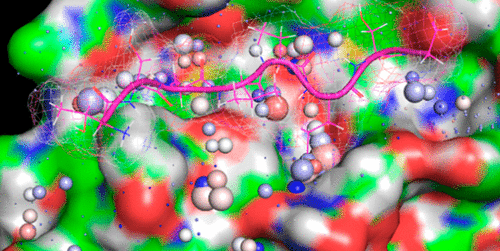
The SARS-CoV-2 main protease (Mpro) is of major interest as an antiviral drug target. Structure-based virtual screening efforts, fueled by a growing list of apo and inhibitor-bound SARS-CoV/CoV-2 Mpro crystal structures, are underway in many laboratories. However, little is known about the dynamic enzyme mechanism, which is needed to inform both assay development and structure-based inhibitor design. Here, we apply biodynamics theory to characterize the structural dynamics of substrate-induced Mpro activation under nonequilibrium conditions. The catalytic cycle is governed by concerted dynamic structural rearrangements of domain 3 and the m-shaped loop (residues 132–147) on which Cys145 (comprising the thiolate nucleophile and half of the oxyanion hole) and Gly143 (comprising the second half of the oxyanion hole) reside. In particular, we observed the following: (1) Domain 3 undergoes dynamic rigid-body rotation about the domain 2–3 linker, alternately visiting two primary conformational states (denoted as M1pro ↔ M2pro); (2) The Gly143-containing crest of the m-shaped loop undergoes up and down translations caused by conformational changes within the rising stem of the loop (Lys137–Asn142) in response to domain 3 rotation and dimerization (denoted as M1/downpro ↔ 2·M2/uppro) (noting that the Cys145-containing crest is fixed in the up position). We propose that substrates associate to the M1/downpro state, which promotes the M2/downpro state, dimerization (denoted as 2·M2/uppro–substrate), and catalysis. Here, we explore the state transitions of Mpro under nonequilibrium conditions, the mechanisms by which they are powered, and the implications thereof for efficacious inhibition under in vivo conditions.
中文翻译:

用生物动力学理论探究冠状病毒主要蛋白酶的动态结构-功能和无结构能的关系
SARS-CoV-2主蛋白酶(M pro)作为抗病毒药物靶标引起了人们的极大兴趣。在许多实验室中,越来越多的脱辅基和结合抑制剂的SARS-CoV / CoV-2 M前晶体结构推动了基于结构的虚拟筛选工作。但是,对于动态酶机制知之甚少,而动态酶机制对于告知化验开发和基于结构的抑制剂设计都是必需的。在这里,我们应用生物动力学理论来表征非平衡条件下底物诱导的M pro活化的结构动力学。催化循环受协调一致结构域3和m形环(残基132-147)的动态结构重排,Cys145(包含硫醇盐亲核试剂和一半的氧阴离子孔)和Gly143(包含氧阴离子孔的后一半)位于其上。特别是,我们观察到以下:(1)结构域3所经受动态刚体关于域2-3接头旋转,交替地来访的两个主要的构象状态(表示为中号1亲↔中号2亲); (2)响应域3旋转和二聚化(表示为M 1 / down),m形环的含Gly143的波峰经历了环的上升茎(Lys137–Asn142)内构象变化而引起的上下翻译。亲↔2·M 2 /达亲)(请注意,含Cys145的波峰已固定在向上位置)。我们建议底物与M 1 /向下亲状态相关联,从而促进M 2 /向下亲状态,二聚作用(表示为2·M 2 /向上亲基质)和催化作用。在这里,我们探讨了非平衡条件下M pro的状态转变,它们的动力机制及其在体内条件下有效抑制的意义。
更新日期:2020-12-12
中文翻译:

用生物动力学理论探究冠状病毒主要蛋白酶的动态结构-功能和无结构能的关系
SARS-CoV-2主蛋白酶(M pro)作为抗病毒药物靶标引起了人们的极大兴趣。在许多实验室中,越来越多的脱辅基和结合抑制剂的SARS-CoV / CoV-2 M前晶体结构推动了基于结构的虚拟筛选工作。但是,对于动态酶机制知之甚少,而动态酶机制对于告知化验开发和基于结构的抑制剂设计都是必需的。在这里,我们应用生物动力学理论来表征非平衡条件下底物诱导的M pro活化的结构动力学。催化循环受协调一致结构域3和m形环(残基132-147)的动态结构重排,Cys145(包含硫醇盐亲核试剂和一半的氧阴离子孔)和Gly143(包含氧阴离子孔的后一半)位于其上。特别是,我们观察到以下:(1)结构域3所经受动态刚体关于域2-3接头旋转,交替地来访的两个主要的构象状态(表示为中号1亲↔中号2亲); (2)响应域3旋转和二聚化(表示为M 1 / down),m形环的含Gly143的波峰经历了环的上升茎(Lys137–Asn142)内构象变化而引起的上下翻译。亲↔2·M 2 /达亲)(请注意,含Cys145的波峰已固定在向上位置)。我们建议底物与M 1 /向下亲状态相关联,从而促进M 2 /向下亲状态,二聚作用(表示为2·M 2 /向上亲基质)和催化作用。在这里,我们探讨了非平衡条件下M pro的状态转变,它们的动力机制及其在体内条件下有效抑制的意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号