当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Active Site of the Antibacterial Target MraY by Modified Tunicamycins
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-11-09 , DOI: 10.1021/acschembio.0c00423 Jenny Hering 1, 2 , Elin Dunevall 2 , Arjan Snijder 3 , Per-Olof Eriksson 1 , Michael A. Jackson 4 , Trina M. Hartman 4 , Ran Ting 5 , Hongming Chen 5 , Neil P. J. Price 4 , Gisela Brändén 2 , Margareta Ek 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-11-09 , DOI: 10.1021/acschembio.0c00423 Jenny Hering 1, 2 , Elin Dunevall 2 , Arjan Snijder 3 , Per-Olof Eriksson 1 , Michael A. Jackson 4 , Trina M. Hartman 4 , Ran Ting 5 , Hongming Chen 5 , Neil P. J. Price 4 , Gisela Brändén 2 , Margareta Ek 1
Affiliation
|
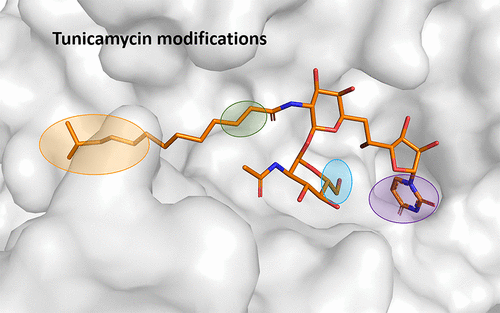
The alarming growth of antibiotic resistance that is currently ongoing is a serious threat to human health. One of the most promising novel antibiotic targets is MraY (phospho-MurNAc-pentapeptide-transferase), an essential enzyme in bacterial cell wall synthesis. Through recent advances in biochemical research, there is now structural information available for MraY, and for its human homologue GPT (GlcNAc-1-P-transferase), that opens up exciting possibilities for structure-based drug design. The antibiotic compound tunicamycin is a natural product inhibitor of MraY that is also toxic to eukaryotes through its binding to GPT. In this work, we have used tunicamycin and modified versions of tunicamycin as tool compounds to explore the active site of MraY and to gain further insight into what determines inhibitor potency. We have investigated tunicamycin variants where the following motifs have been modified: the length and branching of the tunicamycin fatty acyl chain, the saturation of the fatty acyl chain, the 6″-hydroxyl group of the GlcNAc ring, and the ring structure of the uracil motif. The compounds are analyzed in terms of how potently they bind to MraY, inhibit the activity of the enzyme, and affect the protein thermal stability. Finally, we rationalize these results in the context of the protein structures of MraY and GPT.
中文翻译:

修饰的衣霉素探索抗菌靶标MraY的活性位点
当前持续令人担忧的抗生素耐药性增长是对人类健康的严重威胁。最有希望的新型抗生素靶标之一是MraY(磷酸-MurNAc-五肽转移酶),它是细菌细胞壁合成中必不可少的酶。通过生化研究的最新进展,现在可为MraY及其人类同源物GPT(GlcNAc-1-P-转移酶)提供结构信息,这为基于结构的药物设计开辟了令人兴奋的可能性。抗生素化合物衣霉素是MraY的天然产物抑制剂,通过与GPT结合也对真核生物有毒。在这项工作中,我们使用了衣霉素和衣霉素的改良版作为工具化合物来探索MraY的活性位点,并进一步了解决定抑制剂效力的因素。我们研究了衣霉素变体,其中以下基序已被修饰:衣霉素脂肪酰基链的长度和分支,脂肪酰基链的饱和度,GlcNAc环的6''-羟基和尿嘧啶的环结构主题。根据这些化合物与MraY结合的强度,抑制酶的活性以及影响蛋白质热稳定性的方面来分析这些化合物。最后,我们在MraY和GPT的蛋白质结构的背景下合理化这些结果。抑制酶的活性,并影响蛋白质的热稳定性。最后,我们在MraY和GPT的蛋白质结构的背景下合理化这些结果。抑制酶的活性,并影响蛋白质的热稳定性。最后,我们在MraY和GPT的蛋白质结构的背景下合理化这些结果。
更新日期:2020-11-21
中文翻译:

修饰的衣霉素探索抗菌靶标MraY的活性位点
当前持续令人担忧的抗生素耐药性增长是对人类健康的严重威胁。最有希望的新型抗生素靶标之一是MraY(磷酸-MurNAc-五肽转移酶),它是细菌细胞壁合成中必不可少的酶。通过生化研究的最新进展,现在可为MraY及其人类同源物GPT(GlcNAc-1-P-转移酶)提供结构信息,这为基于结构的药物设计开辟了令人兴奋的可能性。抗生素化合物衣霉素是MraY的天然产物抑制剂,通过与GPT结合也对真核生物有毒。在这项工作中,我们使用了衣霉素和衣霉素的改良版作为工具化合物来探索MraY的活性位点,并进一步了解决定抑制剂效力的因素。我们研究了衣霉素变体,其中以下基序已被修饰:衣霉素脂肪酰基链的长度和分支,脂肪酰基链的饱和度,GlcNAc环的6''-羟基和尿嘧啶的环结构主题。根据这些化合物与MraY结合的强度,抑制酶的活性以及影响蛋白质热稳定性的方面来分析这些化合物。最后,我们在MraY和GPT的蛋白质结构的背景下合理化这些结果。抑制酶的活性,并影响蛋白质的热稳定性。最后,我们在MraY和GPT的蛋白质结构的背景下合理化这些结果。抑制酶的活性,并影响蛋白质的热稳定性。最后,我们在MraY和GPT的蛋白质结构的背景下合理化这些结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号