当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adipose stem cell secretome markedly improves rodent heart and human induced pluripotent stem cell‐derived cardiomyocyte recovery from cardioplegic transport solution exposure
STEM CELLS ( IF 5.2 ) Pub Date : 2020-11-07 , DOI: 10.1002/stem.3296 Bradley W Ellis 1 , Dmitry O Traktuev 2, 3 , Stephanie Merfeld-Clauss 2, 3 , Uryan Isik Can 4 , Meijing Wang 5 , Ray Bergeron 2 , Pinar Zorlutuna 1, 4 , Keith L March 2, 3
STEM CELLS ( IF 5.2 ) Pub Date : 2020-11-07 , DOI: 10.1002/stem.3296 Bradley W Ellis 1 , Dmitry O Traktuev 2, 3 , Stephanie Merfeld-Clauss 2, 3 , Uryan Isik Can 4 , Meijing Wang 5 , Ray Bergeron 2 , Pinar Zorlutuna 1, 4 , Keith L March 2, 3
Affiliation
|
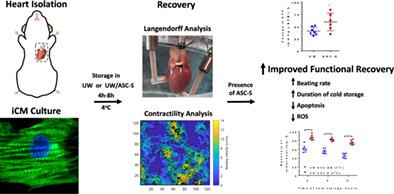
Heart transplantation is a life‐saving therapy for end‐stage organ failure. Organ deterioration during transportation limits storage to 4 hours, limiting hearts available. Approaches ameliorating organ damage could increase the number of hearts acceptable for transplantation. Prior studies show that adipose‐derived stem/stromal cell secretome (ASC‐S) rescues tissues from postischemic damage in vivo. This study tested whether ASC‐S preserved the function of mouse hearts and human induced pluripotent stem cell‐derived cardiomyocytes (iCM) exposed to organ transportation and transplantation conditions. Hearts were subjected to cold University of Wisconsin (UW) cardioplegic solution ± ASC‐S for 6 hours followed by analysis using the Langendorff technique. In parallel, the effects of ASC‐S on the recovery of iCM from UW solution were examined when provided either during or after cold cardioplegia. Exposure of hearts and iCM to UW deteriorated contractile activity and caused cell apoptosis, worsening in iCM as a function of exposure time; these were ameliorated by augmenting with ASC‐S. Silencing of superoxide dismutase 3 and catalase expression prior to secretome generation compromised the ASC‐S cardiomyocyte‐protective effects. In this study, a novel in vitro iCM model was developed to complement a rodent heart model in assessing efficacy of approaches to improve cardiac preservation. ASC‐S displays strong cardioprotective activity on iCM either with or following cold cardioplegia. This effect is associated with ASC‐S‐mediated cellular clearance of reactive oxygen species. The effect of ASC‐S on the temporal recovery of iCM function supports the possibility of lengthening heart storage by augmenting cardioplegic transport solution with ASC‐S, expanding the pool of hearts for transplantation.
中文翻译:

脂肪干细胞分泌组显着改善啮齿动物心脏和人类诱导的多能干细胞衍生的心肌细胞从心脏停搏液暴露中的恢复
心脏移植是终末期器官衰竭的救命疗法。运输过程中的器官退化将储存时间限制为 4 小时,从而限制了可用的心脏。改善器官损伤的方法可以增加移植可接受的心脏数量。先前的研究表明,脂肪来源的干/基质细胞分泌组 (ASC-S) 可以在体内拯救组织免受缺血后损伤。本研究测试了 ASC-S 是否保留了暴露于器官运输和移植条件的小鼠心脏和人类诱导的多能干细胞衍生心肌细胞 (iCM) 的功能。心脏经威斯康星大学 (UW) 心脏停搏液 ± ASC-S 冷处理 6 小时,然后使用 Langendorff 技术进行分析。在平行下,在冷心脏停搏期间或之后提供 ASC-S 对从 UW 溶液中恢复 iCM 的影响进行了检查。心脏和 iCM 暴露于 UW 会降低收缩活性并导致细胞凋亡,iCM 会随着暴露时间的变化而恶化;这些通过增加 ASC-S 得到改善。在分泌组生成之前沉默超氧化物歧化酶 3 和过氧化氢酶的表达会损害 ASC-S 心肌细胞的保护作用。在这项研究中,开发了一种新的体外 iCM 模型来补充啮齿动物心脏模型,以评估改善心脏保存的方法的功效。ASC-S 对 iCM 显示出强烈的心脏保护活性,无论是在冷心脏停搏后还是在冷停搏后。这种效应与 ASC-S 介导的活性氧物质的细胞清除有关。
更新日期:2020-11-07
中文翻译:

脂肪干细胞分泌组显着改善啮齿动物心脏和人类诱导的多能干细胞衍生的心肌细胞从心脏停搏液暴露中的恢复
心脏移植是终末期器官衰竭的救命疗法。运输过程中的器官退化将储存时间限制为 4 小时,从而限制了可用的心脏。改善器官损伤的方法可以增加移植可接受的心脏数量。先前的研究表明,脂肪来源的干/基质细胞分泌组 (ASC-S) 可以在体内拯救组织免受缺血后损伤。本研究测试了 ASC-S 是否保留了暴露于器官运输和移植条件的小鼠心脏和人类诱导的多能干细胞衍生心肌细胞 (iCM) 的功能。心脏经威斯康星大学 (UW) 心脏停搏液 ± ASC-S 冷处理 6 小时,然后使用 Langendorff 技术进行分析。在平行下,在冷心脏停搏期间或之后提供 ASC-S 对从 UW 溶液中恢复 iCM 的影响进行了检查。心脏和 iCM 暴露于 UW 会降低收缩活性并导致细胞凋亡,iCM 会随着暴露时间的变化而恶化;这些通过增加 ASC-S 得到改善。在分泌组生成之前沉默超氧化物歧化酶 3 和过氧化氢酶的表达会损害 ASC-S 心肌细胞的保护作用。在这项研究中,开发了一种新的体外 iCM 模型来补充啮齿动物心脏模型,以评估改善心脏保存的方法的功效。ASC-S 对 iCM 显示出强烈的心脏保护活性,无论是在冷心脏停搏后还是在冷停搏后。这种效应与 ASC-S 介导的活性氧物质的细胞清除有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号