Journal of Structural Biology ( IF 3 ) Pub Date : 2020-11-08 , DOI: 10.1016/j.jsb.2020.107662 Lorenzo Soini 1 , Seppe Leysen 2 , Jeremy Davis 3 , Christian Ottmann 4
|
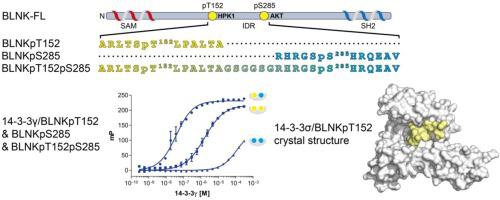
B-cell linker protein (BLNK) is an adaptor protein that orchestrates signalling downstream of B-cell receptors. It has been reported to undergo proteasomal degradation upon binding to 14-3-3 proteins. Here, we report the first biophysical and structural study of this protein-protein interaction (PPI). Specifically, we investigated the binding of mono- and di- phosphorylated BLNK peptides to 14-3-3 using fluorescent polarization (FP) and isothermal titration calorimetry assays (ITC). Our results suggest that BLNK interacts with 14-3-3 according to the gatekeeper model, where HPK1 mediated phosphorylation of Thr152 (pT152) allows BLNK anchoring to 14-3-3, and an additional phosphorylation of Ser285 (pS285) by AKT, then further improves the affinity. Finally, we have also solved a crystal structure of the BLNKpT152 peptide bound to 14-3-3σ. These findings could serve as important tool for compound discovery programs aiming to modulate this interaction with 14-3-3.
中文翻译:

BLNK 与 14-3-3 蛋白相互作用的生物物理和结构分析
B 细胞连接蛋白 (BLNK) 是一种衔接蛋白,可协调 B 细胞受体下游的信号传导。据报道,与 14-3-3 蛋白结合后会发生蛋白酶体降解。在这里,我们报告了这种蛋白质-蛋白质相互作用 (PPI) 的首次生物物理和结构研究。具体而言,我们使用荧光偏振 (FP) 和等温滴定量热分析 (ITC) 研究了单磷酸化和双磷酸化 BLNK 肽与 14-3-3 的结合。我们的结果表明,根据守门人模型,BLNK 与 14-3-3 相互作用,其中 HPK1 介导的 Thr152(pT152)磷酸化允许 BLNK 锚定到 14-3-3,并通过 AKT 对 Ser285(pS285)进行额外的磷酸化,然后进一步提高了亲和力。最后,我们还解析了与 14-3-3σ 结合的 BLNKpT152 肽的晶体结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号