Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-11-09 , DOI: 10.1016/j.bmc.2020.115853 Peng-Chao Huo 1 , Qing Hu 2 , Sheng Shu 3 , Qi-Hang Zhou 2 , Rong-Jing He 2 , Jie Hou 4 , Xiao-Qing Guan 2 , Dong-Zhu Tu 2 , Xu-Dong Hou 4 , Peng Liu 3 , Nan Zhang 5 , Zhi-Guo Liu 3 , Guang-Bo Ge 2
|
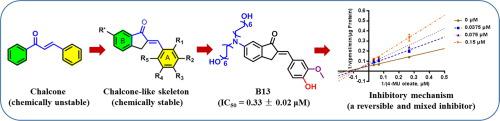
Pancreatic lipase (PL), a crucial enzyme responsible for hydrolysis of dietary lipids, has been validated as a key therapeutic target to prevent and treat obesity-associated metabolic disorders. Herein, we report the design, synthesis and biological evaluation of a series of chalcone-like compounds as potent and reversible PL inhibitors. Following two rounds of structural modifications at both A and B rings of a chalcone-like skeleton, structure-PL inhibition relationships of the chalcone-like compounds were studied, while the key substituents that would be beneficial for PL inhibition were revealed. Among all tested chalcone-like compounds, compound B13 (a novel chalcone-like compound bearing two long carbon chains) displayed the most potent PL inhibition activity, with an IC50 value of 0.33 μM. Inhibition kinetic analyses demonstrated that B13 could potently inhibit PL-mediated 4-MUO hydrolysis in a mixed inhibition manner, with the Ki value of 0.12 μM. Molecular docking simulations suggested that B13 could tightly bind on PL at both the catalytic site and a non-catalytic site that was located on the surface of PL, which was consistent with the mixed inhibition mode of this agent. In addition, B13 displayed excellent stability in artificial gastrointestinal fluids and good metabolic stability in human liver preparations. Collectively, our findings suggested that chalcone-like compounds were good choices for design and development of orally administrated PL inhibitors, while B13 could be served as a promising lead compound to develop novel anti-obesity agents via targeting on PL.
中文翻译:

新型查耳酮类化合物作为有效且可逆的胰脂肪酶抑制剂的设计、合成和生物学评价
胰腺脂肪酶 (PL) 是一种负责水解膳食脂质的关键酶,已被证实是预防和治疗肥胖相关代谢紊乱的关键治疗靶点。在此,我们报告了一系列作为有效和可逆 PL 抑制剂的查耳酮类化合物的设计、合成和生物学评价。在类查尔酮骨架的 A 环和 B 环处进行两轮结构修饰后,研究了类查耳酮化合物的结构-PL 抑制关系,同时揭示了有利于 PL 抑制的关键取代基。在所有测试的查尔酮类化合物中,化合物B13(一种新型查尔酮类化合物,带有两条长碳链)显示出最有效的 PL 抑制活性,IC 500.33 μM 的值。抑制动力学分析表明,B13可以以混合抑制方式有效抑制 PL 介导的 4-MUO 水解,K i值为 0.12 μM。分子对接模拟表明,B13可以在催化位点和位于 PL 表面的非催化位点与 PL 紧密结合,这与该试剂的混合抑制模式一致。此外,B13在人工胃肠液中表现出优异的稳定性,在人肝制剂中表现出良好的代谢稳定性。总的来说,我们的研究结果表明,查耳酮类化合物是设计和开发口服 PL 抑制剂的不错选择,而B13可作为一种有前景的先导化合物,通过靶向 PL开发新型抗肥胖剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号