当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lighting Up the Thrombin-Binding Aptamer G-Quadruplex with an Internal Cyanine-Indole-Quinolinium Nucleobase Surrogate. Direct Fluorescent Intensity Readout for Thrombin Binding without Topology Switching
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-11-06 , DOI: 10.1021/acs.bioconjchem.0c00530 Micaela D Gray 1 , Prashant S Deore 1 , Andrew J Chung 1 , Abigail J Van Riesen 1 , Richard A Manderville 1 , Preethi Seelam Prabhakar 2 , Stacey D Wetmore 2
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-11-06 , DOI: 10.1021/acs.bioconjchem.0c00530 Micaela D Gray 1 , Prashant S Deore 1 , Andrew J Chung 1 , Abigail J Van Riesen 1 , Richard A Manderville 1 , Preethi Seelam Prabhakar 2 , Stacey D Wetmore 2
Affiliation
|
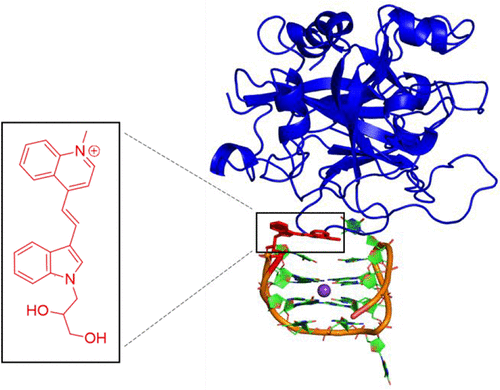
Fluorescent nucleobases represent an important class of molecular reporters of nucleic acid interactions. In this work, the advantages of utilizing a noncanonical fluorescent nucleobase surrogate for monitoring thrombin binding by the 15-mer thrombin binding aptamer (TBA) is presented. TBA folds into an antiparallel G-quadruplex (GQ) with loop thymidine (T) residues interacting directly with the protein in the thrombin–TBA complex. In the free GQ, T3 is solvent-exposed and does not form canonical base-pairs within the antiparallel GQ motif. Upon thrombin binding, T3 interacts directly with a hydrophobic protein binding pocket. Replacing T3 with a cyanine-indole-quinolinium (4QI) hemicyanine dye tethered to an acyclic 1,2-propanediol linker is shown to have minimal impact on GQ stability and structure with the internal 4QI displaying a 40-fold increase in emission intensity at 586 nm (excitation 508 nm) compared to the free dye in solution. Molecular dynamics (MD) simulations demonstrate that the 4QI label π-stacks with T4 and T13 within the antiparallel GQ fold, which is supported by strong energy transfer (ET) fluorescence from the GQ (donor) to the 4QI label (acceptor). Thrombin binding to 4QI-TBA diminishes π-stacking interactions between 4QI and the GQ structure to cause a turn-off emission intensity response with an apparent dissociation constant (Kd) of 650 nM and a limit of detection (LoD) of 150 nM. These features highlight the utility of internal noncanonical fluorescent surrogates for monitoring protein binding by GQ-folding aptamers in the absence of DNA topology switching.
中文翻译:

用内部花菁-吲哚-喹啉鎓核碱基替代物点亮凝血酶结合适体G-四联体。凝血酶结合的直接荧光强度读数,无需拓扑切换
荧光核碱基代表一类重要的核酸相互作用分子报告分子。在这项工作中,提出了利用非规范性荧光核碱基替代物通过15-mer凝血酶结合适体(TBA)监测凝血酶结合的优势。TBA折叠成带有环胸腺嘧啶(T)残基的反平行G四联体(GQ),后者直接与凝血酶-TBA复合物中的蛋白质相互作用。在游离GQ中,T3暴露于溶剂中,并且在反平行GQ基序内不形成规范的碱基对。凝血酶结合后,T3与疏水蛋白结合袋直接相互作用。用拴在无环1上的花青-吲哚-喹啉(4QI)半花青染料代替T3,与溶液中的游离染料相比,2-丙二醇接头对GQ稳定性和结构的影响最小,内部4QI在586 nm(激发508 nm)下的发射强度增加了40倍。分子动力学(MD)模拟表明,4QI标记的π-堆栈在反平行GQ折叠内具有T4和T13,这由从GQ(供体)到4QI标记(受体)的强能量转移(ET)荧光支持。凝血酶与4QI-TBA的结合减少了4QI与GQ结构之间的π堆积相互作用,从而导致了具有明显解离常数的截止发射强度响应(分子动力学(MD)模拟表明,4QI标记的π-堆栈在反平行GQ折叠内具有T4和T13,这由从GQ(供体)到4QI标记(受体)的强能量转移(ET)荧光支持。凝血酶与4QI-TBA的结合减少了4QI与GQ结构之间的π堆积相互作用,从而导致了具有明显解离常数的截止发射强度响应(分子动力学(MD)模拟表明,4QI标记的π-堆栈在反平行GQ折叠内具有T4和T13,这由从GQ(供体)到4QI标记(受体)的强能量转移(ET)荧光支持。凝血酶与4QI-TBA的结合减少了4QI与GQ结构之间的π堆积相互作用,从而导致了具有明显解离常数的截止发射强度响应(K d)为650 nM,检测极限(LoD)为150 nM。这些特征突出了内部非规范荧光替代物在不进行DNA拓扑转换的情况下通过GQ折叠适体监测蛋白质结合的实用性。
更新日期:2020-11-18
中文翻译:

用内部花菁-吲哚-喹啉鎓核碱基替代物点亮凝血酶结合适体G-四联体。凝血酶结合的直接荧光强度读数,无需拓扑切换
荧光核碱基代表一类重要的核酸相互作用分子报告分子。在这项工作中,提出了利用非规范性荧光核碱基替代物通过15-mer凝血酶结合适体(TBA)监测凝血酶结合的优势。TBA折叠成带有环胸腺嘧啶(T)残基的反平行G四联体(GQ),后者直接与凝血酶-TBA复合物中的蛋白质相互作用。在游离GQ中,T3暴露于溶剂中,并且在反平行GQ基序内不形成规范的碱基对。凝血酶结合后,T3与疏水蛋白结合袋直接相互作用。用拴在无环1上的花青-吲哚-喹啉(4QI)半花青染料代替T3,与溶液中的游离染料相比,2-丙二醇接头对GQ稳定性和结构的影响最小,内部4QI在586 nm(激发508 nm)下的发射强度增加了40倍。分子动力学(MD)模拟表明,4QI标记的π-堆栈在反平行GQ折叠内具有T4和T13,这由从GQ(供体)到4QI标记(受体)的强能量转移(ET)荧光支持。凝血酶与4QI-TBA的结合减少了4QI与GQ结构之间的π堆积相互作用,从而导致了具有明显解离常数的截止发射强度响应(分子动力学(MD)模拟表明,4QI标记的π-堆栈在反平行GQ折叠内具有T4和T13,这由从GQ(供体)到4QI标记(受体)的强能量转移(ET)荧光支持。凝血酶与4QI-TBA的结合减少了4QI与GQ结构之间的π堆积相互作用,从而导致了具有明显解离常数的截止发射强度响应(分子动力学(MD)模拟表明,4QI标记的π-堆栈在反平行GQ折叠内具有T4和T13,这由从GQ(供体)到4QI标记(受体)的强能量转移(ET)荧光支持。凝血酶与4QI-TBA的结合减少了4QI与GQ结构之间的π堆积相互作用,从而导致了具有明显解离常数的截止发射强度响应(K d)为650 nM,检测极限(LoD)为150 nM。这些特征突出了内部非规范荧光替代物在不进行DNA拓扑转换的情况下通过GQ折叠适体监测蛋白质结合的实用性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号