当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quassinoids from the Root Barks of Ailanthus altissima: Isolation, Configurational Assignment, and Cytotoxic Activities
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-11-06 , DOI: 10.1002/cjoc.202000558 Ye‐Qing Du 1 , Ming Bai 1 , Xiao‐Qi Yu 1 , Tian‐Ming Lv 1 , Bin Lin 2 , Xiao‐Xiao Huang 1 , Shao‐Jiang Song 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-11-06 , DOI: 10.1002/cjoc.202000558 Ye‐Qing Du 1 , Ming Bai 1 , Xiao‐Qi Yu 1 , Tian‐Ming Lv 1 , Bin Lin 2 , Xiao‐Xiao Huang 1 , Shao‐Jiang Song 1
Affiliation
|
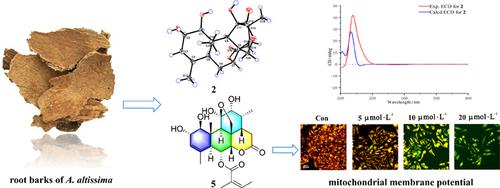
Quassinoids, a class of highly oxygenated triterpenes, have been isolated as bitter principles from the plants of Simaroubaceae family. Five new quassinoids, chouchunlactone A—E (1—5), and two known ones were identified from the root barks of Ailanthus altissima Swingle (Simaroubaceae). The chemical structures of the new compounds were deduced by spectroscopic data analyses, NMR calculations and DP4+ probability analysis. The absolute configurations were defined by comparison of their experimental and calculated electronic circular dichroism (ECD) spectra data, and compounds 1—2 were further confirmed by single‐crystal X‐ray diffraction. Biologically, compound 5 showed potencies equivalent to sorafenib (IC50 value, 9.70 μmol·L–1) against HepG2 cells. The Hoechst 33342 staining, JC‐1 fluorescent dye and Annexin V/PI analysis studies demonstrated that 5 can induce apoptosis and attenuate mitochondrial membrane potential (MMP) in HepG2 cells.
中文翻译:

臭椿根皮中的类拟南芥:分离,构型分配和细胞毒活性。
从苦瓜科植物中分离出苦味素类苦味素是一类高度氧化的三萜。五个新quassinoids,chouchunlactone A-E (1 - 5),以及两个公知的是从的根皮识别臭椿欢乐之(苦木科)。通过光谱数据分析,NMR计算和DP4 +概率分析推导了新化合物的化学结构。的绝对构型可以通过试验的和计算电子圆二色性(ECD)光谱数据的比较所定义的化合物,和1 - 2通过单晶X射线衍射进一步证实。从生物学上讲,化合物5对HepG2细胞显示出等效于索拉非尼的效力(IC 50值为9.70μmol·L –1)。Hoechst 33342染色,JC-1荧光染料和Annexin V / PI分析研究表明5可以诱导HepG2细胞凋亡并减弱线粒体膜电位(MMP)。
更新日期:2020-11-06

中文翻译:

臭椿根皮中的类拟南芥:分离,构型分配和细胞毒活性。
从苦瓜科植物中分离出苦味素类苦味素是一类高度氧化的三萜。五个新quassinoids,chouchunlactone A-E (1 - 5),以及两个公知的是从的根皮识别臭椿欢乐之(苦木科)。通过光谱数据分析,NMR计算和DP4 +概率分析推导了新化合物的化学结构。的绝对构型可以通过试验的和计算电子圆二色性(ECD)光谱数据的比较所定义的化合物,和1 - 2通过单晶X射线衍射进一步证实。从生物学上讲,化合物5对HepG2细胞显示出等效于索拉非尼的效力(IC 50值为9.70μmol·L –1)。Hoechst 33342染色,JC-1荧光染料和Annexin V / PI分析研究表明5可以诱导HepG2细胞凋亡并减弱线粒体膜电位(MMP)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号