Microporous and Mesoporous Materials ( IF 5.2 ) Pub Date : 2020-11-06 , DOI: 10.1016/j.micromeso.2020.110744 Gion Calzaferri , Samuel H. Gallagher , Dominik Brühwiler
|
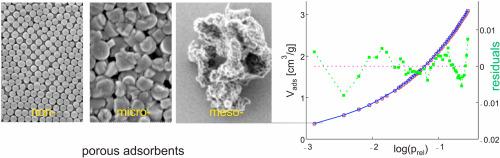
Analysis of multiple equilibria of compounds with different coordination sites is extended to the description of adsorption isotherms with focus on the low relative pressure range. The entropy evolution is described using the particle distribution theory which also holds for adsorbents consisting of materials bearing more than one type of sites and applies for the condition that the adsorptive-adsorbent binding strength is larger than the adsorptive-adsorbate binding strength, so that monolayer coverage is favored. This allows to accurately determine the adsorption enthalpy. No assumption concerning the growth mechanism and specifics regarding the structure of the surface is needed. We find on a rigorous basis that this leads to Langmuir's equation for each site independently, that the total fractional amount of bound adsorptive can be described as a linear combination of individual Langmuir isotherms, and that such a linear combination has never the shape of the original Langmuir isotherms. The results are successfully applied to argon and nitrogen adsorption isotherms of nonporous, microporous, and mesoporous adsorbents which allows comparing systems for which the properties of the active surface span a large range. We observe that all experimental data can accurately be described by means of a linear combination of two Langmuir isotherms in the low relative pressure range up to a coverage of 60%–95%. This means that the shape of all adsorption isotherms is essentially determined by the entropy decrease with increasing coverage. The two site interactions involved exhibit substantially different adsorption enthalpies. Interestingly the Ar enthalpy of adsorption of the sites 1 for the Stöber-type silica and of the three investigated MCM-41 adsorbents (with pore size of 2.7 nm, 4.1 nm, and 4.4 nm) are similar, namely −11 kJ/mol. The situation is analogous for the enthalpy of adsorption for the sites 2, which amounts to −8 kJ/mol. A significantly larger enthalpy of adsorption for the sites 1, namely −14.3 kJ/mol, and for the sites 2 is measured for potassium zeolite L thus reflecting the more polar nature of this adsorbent. The measured specific surface area for these samples ranges from 14 m2/g for the Stöber-type silica up to 1100 m2/g for the MCM-41(4.1 nm) adsorbent. The information provided by the lc2-L (linear combination of 2 Langmuir functions) analysis allows calculating the evolution of the coverage of site 1 and of site 2 as a function of increasing pressure. The inflection points of the isotherms, which mark the point where the curvature changes sign, were determined by numerically evaluating the second derivatives which vanish at this point and are compared with values obtained using BET analysis.
中文翻译:

多重平衡中的熵。无孔,微孔和中孔材料的氩和氮吸附等温线
具有不同配位点的化合物的多重平衡分析扩展到吸附等温线的描述,重点是较低的相对压力范围。使用粒子分布理论描述熵的演化,该理论也适用于由具有一种以上类型位点的材料组成的吸附剂,并适用于吸附-吸附剂结合强度大于吸附-吸附剂结合强度的条件,因此单层覆盖率较高。这允许精确地确定吸附焓。不需要关于生长机理的假设和关于表面结构的细节。在严格的基础上,我们发现每个站点独立地得出Langmuir方程,吸附吸附剂的总分数可以描述为各个Langmuir等温线的线性组合,并且这种线性组合永远不会具有原始Langmuir等温线的形状。该结果成功地应用于无孔,微孔和中孔吸附剂的氩气和氮气吸附等温线,从而可以比较活性表面性质跨度较大的系统。我们观察到,所有实验数据都可以通过在低相对压力范围内高达60%–95%的两个Langmuir等温线的线性组合来准确描述。这意味着,所有吸附等温线的形状基本上取决于随覆盖率增加而降低的熵。所涉及的两个位点相互作用表现出明显不同的吸附焓。Stöber型二氧化硅的位点1和三种研究的MCM-41吸附剂(孔径分别为2.7 nm,4.1 nm和4.4 nm)相似,即-11 kJ / mol。这种情况类似于吸附的焓对于位点2,其为-8 kJ / mol。明显更大的吸附焓 对于位点1,即-14.3 kJ / mol,和 对于位点2,测定了K沸石的L,因此反映出该吸附剂的极性更大。这些样品的比表面积从Stöber型二氧化硅的14 m 2 / g到MCM-41(4.1 nm)吸附剂的1100 m 2 / g不等。lc2-L(2个Langmuir函数的线性组合)分析提供的信息允许计算站点1和站点2的覆盖率随压力增加而变化的情况。通过对在该点消失的二阶导数进行数值评估来确定等温线的拐点,这些拐点标记了曲率改变符号的点,并与使用BET分析获得的值进行比较。


























 京公网安备 11010802027423号
京公网安备 11010802027423号