当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Green and Sustainable Manufacturing Process for Gefapixant Citrate (MK-7264) Part 4: Formylation–Cyclization as a Flow–Batch Process Leads to Significant Improvements in Process Mass Intensity (PMI) and CO Generated versus the Batch–Batch Process
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.oprd.0c00252 Douglas A. L. Otte 1 , Kallol Basu 1 , Lisa Jellett 1 , Michael Whittington 1 , Glenn Spencer 1 , Matthew Burris 1 , Emily B. Corcoran 1 , Kevin Stone 1 , Jarod Nappi 1 , Rebecca A. Arvary 1 , David Donoghue 2 , Hong Ren 1 , Kevin M. Maloney 1 , John R. Naber 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.oprd.0c00252 Douglas A. L. Otte 1 , Kallol Basu 1 , Lisa Jellett 1 , Michael Whittington 1 , Glenn Spencer 1 , Matthew Burris 1 , Emily B. Corcoran 1 , Kevin Stone 1 , Jarod Nappi 1 , Rebecca A. Arvary 1 , David Donoghue 2 , Hong Ren 1 , Kevin M. Maloney 1 , John R. Naber 1
Affiliation
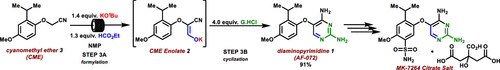
|
Gefapixant citrate (MK-7264) is a P2X3 antagonist for the treatment of chronic cough. The second generation manufacturing route developed for the Step 3A/3B formylation–cyclization reaction to generate the key intermediate diaminopyrimidine (1) (AF-072) required a significant excess of ethyl formate (EF), potassium tert-butoxide (KOt-Bu), and guanidine•HCl (G•HCl) when both steps were run as batch processes. It was imperative to develop an alternative process that required less of each reagent and generated less carbon monoxide byproducts, as the annual production of the final active pharmaceutical ingredient (API) is expected to be over 50 MT. In addition, the second generation process was misaligned with our company’s strategy of having the best science in place at the first regulatory filing. The final flow–batch process described herein, which features a flow-based formylation combined with a batch cyclization, has been performed on a 500 kg scale and now requires 35% less EF (leading to a 70% reduction in waste carbon monoxide), 38% less KOt-Bu, and 50% less G•HCl. These improvements, along with a twofold increase in concentration, have resulted in a 54% reduction in the step process mass intensity (step-PMI) from the second generation two-step batch–batch process (PMI of 17.16) to the flow–batch process (PMI of 7.86), without sacrificing reaction performance.
中文翻译:

柠檬酸Gefapixant(MK-7264)的绿色可持续制造工艺的开发,第4部分:作为分批工艺的甲酰化-环化工艺,与分批工艺相比,工艺质量强度(PMI)和产生的CO有了显着改善
柠檬酸Gefapixant(MK-7264)是用于治疗慢性咳嗽的P2X3拮抗剂。为步骤3A / 3B甲酰化-环化反应开发的第二代生产路线,以生成关键的中间体二氨基嘧啶(1)(AF-072),需要大量过量的甲酸乙酯(EF),叔丁醇钾(KO t-两个步骤都以批处理方式运行时,加入Bu)和胍·HCl(G·HCl)。由于最终活性药物成分(API)的年产量预计超过50吨,因此必须开发一种替代方法,该方法需要较少的每种试剂并产生较少的一氧化碳副产物。此外,第二代流程与我们公司在第一份监管备案中拥有最佳科学依据的战略不符。本文所述的最终分批生产工艺以流式甲酰化和间歇环化相结合,已在500公斤规模上进行,现在所需的EF降低了35%(导致一氧化碳废料减少了70%), KO t-减少38%Bu和少50%的G•HCl。这些改进以及浓度的两倍提高,使得从第二代两步分批处理(17.16的PMI)到分流批处理的阶梯式过程质量强度(step-PMI)降低了54%。过程(PMI为7.86),而不会牺牲反应性能。
更新日期:2020-11-21
中文翻译:

柠檬酸Gefapixant(MK-7264)的绿色可持续制造工艺的开发,第4部分:作为分批工艺的甲酰化-环化工艺,与分批工艺相比,工艺质量强度(PMI)和产生的CO有了显着改善
柠檬酸Gefapixant(MK-7264)是用于治疗慢性咳嗽的P2X3拮抗剂。为步骤3A / 3B甲酰化-环化反应开发的第二代生产路线,以生成关键的中间体二氨基嘧啶(1)(AF-072),需要大量过量的甲酸乙酯(EF),叔丁醇钾(KO t-两个步骤都以批处理方式运行时,加入Bu)和胍·HCl(G·HCl)。由于最终活性药物成分(API)的年产量预计超过50吨,因此必须开发一种替代方法,该方法需要较少的每种试剂并产生较少的一氧化碳副产物。此外,第二代流程与我们公司在第一份监管备案中拥有最佳科学依据的战略不符。本文所述的最终分批生产工艺以流式甲酰化和间歇环化相结合,已在500公斤规模上进行,现在所需的EF降低了35%(导致一氧化碳废料减少了70%), KO t-减少38%Bu和少50%的G•HCl。这些改进以及浓度的两倍提高,使得从第二代两步分批处理(17.16的PMI)到分流批处理的阶梯式过程质量强度(step-PMI)降低了54%。过程(PMI为7.86),而不会牺牲反应性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号