当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Primer for Pharmaceutical Process Development Chemists and Analysts in Relation to Impurities Perceived to Be Mutagenic or “Genotoxic”
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.oprd.0c00343 David J. Snodin 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.oprd.0c00343 David J. Snodin 1
Affiliation
|
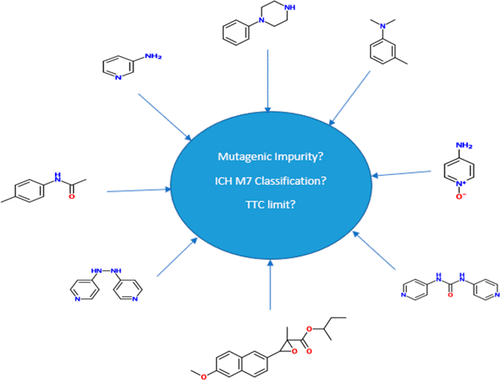
Regulatory guidance on impurities is becoming increasingly comprehensive and complex. The advent of ICH M7(R1) on mutagenic impurities has introduced a significant and sophisticated toxicological component that can easily be underestimated by the unwary. The term “genotoxic impurity” was used in guidelines that predated the current guidance but is now outdated, although it is still often (mis)used in publications on impurities. ICH M7(R1) applies only to mutagenic impurities, which are defined as compounds that are DNA-reactive and test positive in a bacterial reverse mutation assay or are predicted to do so using appropriate in silico structure–activity software. A tentative indication of mutagenic activity is provided by so-called structural alerts, which are certain electrophilic moieties within a chemical structure. It is now well-established that many conventional alerts are associated with a significant number of false-positive predictions of mutagenic potential. Consequently, caution is required when an alert is used to tag a particular impurity as “genotoxic” with no further checks. Such an approach might lead to the development of unnecessarily sensitive impurity assays, which may or may not be a deliberate choice, and possibly to wasted additional process development costs. This review is intended to provide pragmatic guidance on the evaluation of the mutagenicity status of impurities, on the basis of which it is possible to determine appropriate limits. In addition, a series of published examples are reviewed where analytical method development has been compromised by mistakes concerning mutagenicity status and where incorrect mechanistic assumptions have been made regarding the potential for the formation of particular impurities.
中文翻译:

与认为是诱变或“遗传毒性”的杂质有关的制药工艺开发化学家和分析家入门
关于杂质的法规指南变得越来越全面和复杂。ICH M7(R1)在诱变杂质上的出现引入了重要而复杂的毒理学成分,容易被低估的人低估。准则中使用了“遗传毒性杂质”一词,该准则早于当前准则,但现在已经过时,尽管在杂质出版物中仍经常(误用)它。ICH M7(R1)仅适用于诱变杂质,其被定义为具有DNA反应性且在细菌反向突变测定中呈阳性反应的化合物,或预计将使用适当的计算机模拟活性软件进行检测。诱变活性的初步指示是通过所谓的结构警报来提供的,结构警报是化学结构中的某些亲电子部分。现在公认的是,许多常规警报与大量诱变潜力的假阳性预测有关。因此,当使用警报将特定杂质标记为“遗传毒性”而没有进一步检查时,则需要谨慎。这种方法可能会导致不必要的敏感杂质分析方法的开发,这可能是或不是故意的选择,并可能浪费了额外的过程开发成本。本文旨在为评估杂质的致突变性状态提供实用的指导,并以此为基础确定适当的限值。此外,
更新日期:2020-11-21
中文翻译:

与认为是诱变或“遗传毒性”的杂质有关的制药工艺开发化学家和分析家入门
关于杂质的法规指南变得越来越全面和复杂。ICH M7(R1)在诱变杂质上的出现引入了重要而复杂的毒理学成分,容易被低估的人低估。准则中使用了“遗传毒性杂质”一词,该准则早于当前准则,但现在已经过时,尽管在杂质出版物中仍经常(误用)它。ICH M7(R1)仅适用于诱变杂质,其被定义为具有DNA反应性且在细菌反向突变测定中呈阳性反应的化合物,或预计将使用适当的计算机模拟活性软件进行检测。诱变活性的初步指示是通过所谓的结构警报来提供的,结构警报是化学结构中的某些亲电子部分。现在公认的是,许多常规警报与大量诱变潜力的假阳性预测有关。因此,当使用警报将特定杂质标记为“遗传毒性”而没有进一步检查时,则需要谨慎。这种方法可能会导致不必要的敏感杂质分析方法的开发,这可能是或不是故意的选择,并可能浪费了额外的过程开发成本。本文旨在为评估杂质的致突变性状态提供实用的指导,并以此为基础确定适当的限值。此外,

























 京公网安备 11010802027423号
京公网安备 11010802027423号