当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation Mechanism of Thiophosphate Anions in the Liquid-Phase Synthesis of Sulfide Solid Electrolytes Using Polar Aprotic Solvents
Chemistry of Materials ( IF 8.6 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.chemmater.0c03198 Marcela Calpa 1 , Nataly Carolina Rosero-Navarro 2 , Akira Miura 2 , Kota Terai 3 , Futoshi Utsuno 3 , Kiyoharu Tadanaga 2
Chemistry of Materials ( IF 8.6 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.chemmater.0c03198 Marcela Calpa 1 , Nataly Carolina Rosero-Navarro 2 , Akira Miura 2 , Kota Terai 3 , Futoshi Utsuno 3 , Kiyoharu Tadanaga 2
Affiliation
|
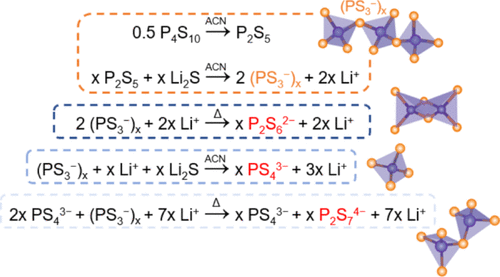
Preparation of sulfide-based Li-ion conductive solid electrolytes by a liquid-phase process has been proposed as an effective route for industrial scaling-up. However, reaction mechanisms in the liquid-phase synthesis or the role of solvents in the reactions is not yet well understood. Here, the reaction between Li2S and P2S5 in the composition of 50:50 mol % mediated by a polar aprotic solvent (acetonitrile) was investigated. The study of the crystal and local structure of the 50Li2S·50P2S5 sample revealed large chemical changes upon crystallization, highlighting the initial formation of a polymer-like (PS3–)n intermediate. On the basis of the (PS3–)n intermediate, the reaction pathways for the formation of P2S62–, PS43–, and P2S74– anions are elucidated. These findings give a deeper insight into the reaction mechanisms governing the liquid-phase synthesis of sulfide solid electrolytes and provide more specific criteria for designing novel materials with superior characteristics through this liquid-phase approach.
中文翻译:

极性非质子溶剂液相合成硫化物固体电解质中硫代磷酸根阴离子的形成机理
已经提出通过液相法制备硫化物基锂离子导电固体电解质作为工业规模化的有效途径。然而,关于液相合成中的反应机理或溶剂在反应中的作用尚不十分了解。在此,研究了由极性非质子溶剂(乙腈)介导的Li 2 S与P 2 S 5在50∶50mol%组成中的反应。对50Li 2 S·50P 2 S 5样品的晶体和局部结构的研究表明,结晶后化学性质发生了较大变化,突出了聚合物样(PS 3 –)n的初始形成。中间。基于(PS 3 –)n中间体,阐明了形成P 2 S 6 2–,PS 4 3–和P 2 S 7 4–阴离子的反应途径。这些发现使人们对控制硫化物固体电解质液相合成的反应机理有了更深入的了解,并为通过这种液相方法设计具有优异特性的新型材料提供了更具体的标准。
更新日期:2020-11-25
中文翻译:

极性非质子溶剂液相合成硫化物固体电解质中硫代磷酸根阴离子的形成机理
已经提出通过液相法制备硫化物基锂离子导电固体电解质作为工业规模化的有效途径。然而,关于液相合成中的反应机理或溶剂在反应中的作用尚不十分了解。在此,研究了由极性非质子溶剂(乙腈)介导的Li 2 S与P 2 S 5在50∶50mol%组成中的反应。对50Li 2 S·50P 2 S 5样品的晶体和局部结构的研究表明,结晶后化学性质发生了较大变化,突出了聚合物样(PS 3 –)n的初始形成。中间。基于(PS 3 –)n中间体,阐明了形成P 2 S 6 2–,PS 4 3–和P 2 S 7 4–阴离子的反应途径。这些发现使人们对控制硫化物固体电解质液相合成的反应机理有了更深入的了解,并为通过这种液相方法设计具有优异特性的新型材料提供了更具体的标准。



























 京公网安备 11010802027423号
京公网安备 11010802027423号