当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Protonation State of a Key Histidine Ligand in the Iron–Quinone Complex of Photosystem II as Revealed by Light-Induced ATR-FTIR Spectroscopy
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-04 , DOI: 10.1021/acs.biochem.0c00810 Masakazu Kimura 1 , Yuki Kato 1 , Takumi Noguchi 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-04 , DOI: 10.1021/acs.biochem.0c00810 Masakazu Kimura 1 , Yuki Kato 1 , Takumi Noguchi 1
Affiliation
|
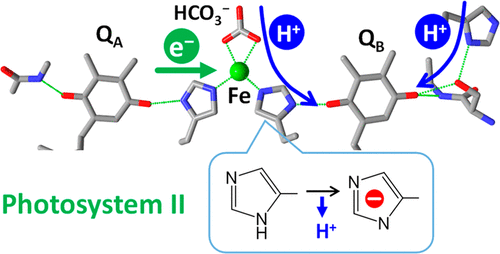
The iron–quinone complex in photosystem II (PSII) consists of the two plastoquinone electron acceptors, QA and QB, and a non-heme iron connecting them. It has been suggested that nearby histidine residues play important roles in the electron and proton transfer reactions of the iron–quinone complex in PSII. In this study, we investigated the protonation/deprotonation reaction of D1-H215, which bridges the non-heme iron and QB, using attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy. Flash-induced Fe2+/Fe3+ ATR-FTIR difference spectra were measured with PSII membranes in the pH range of 5.0–7.5. In the CN stretching region of histidine, the intensity of a negative peak at 1094 cm–1, which was assigned to the deprotonated anion form of D1-H215, increased as the pH increased. Singular-value decomposition analysis provided a component due to deprotonation of D1-H215 with a pKa of ∼5.5 in the Fe3+ state, whereas no component of histidine deprotonation was resolved in the Fe2+ state. This observation supports the previous proposal that D1-H215 is responsible for the proton release upon Fe2+ oxidation [Berthomieu, C., and Hienerwadel, R. (2001) Biochemistry 40, 4044–4052]. The pH dependence of the 13C isotope-edited bands of the bicarbonate ligand to the non-heme iron further showed that deprotonation of bicarbonate to carbonate does not take place at pH <8 in the Fe2+ or Fe3+ state. These results suggest that the putative mechanism of proton transfer to QBH– through D1-H215 and bicarbonate around Fe2+ functions throughout the physiological pH range.
中文翻译:

光诱导的ATR-FTIR光谱揭示了光系统II的铁-醌复合物中关键组氨酸配体的质子化状态
光系统II(PSII)中的铁-醌配合物由两个质体醌电子受体Q A和Q B以及连接它们的非血红素铁组成。有人认为,附近的组氨酸残基在PSII中铁-醌配合物的电子和质子转移反应中起重要作用。在这项研究中,我们使用衰减全反射傅里叶变换红外(ATR-FTIR)光谱技术研究了D1-H215桥接非血红素铁和Q B的质子/去质子反应。闪光诱导的Fe 2+ / Fe 3+ ATR-FTIR差异光谱在5.0-7.5的pH范围内用PSII膜测量。在组氨酸的CN伸展区中,在1094 cm处出现一个负峰强度随着pH值的增加,–1被分配为D1-H215的去质子化阴离子形式。在Fe 3+状态下,奇异值分解分析提供了由于D1-H215的去质子化而ap K a为〜5.5的组分,而在Fe 2+状态下没有组氨酸去质子化的组分。该观察结果支持了先前的建议,即D1-H215负责质子在Fe 2+氧化时的释放[Berthomieu,C.和Hienerwadel,R.(2001)Biochemistry 40,4044–4052]。13的pH依赖性碳酸氢盐配体在非血红素铁上的C同位素编辑谱带进一步显示,在pH <8的Fe 2+或Fe 3+状态下,碳酸氢盐的去质子化反应不会发生。这些结果表明,在整个生理pH范围内,质子通过D1-H215和Fe 2+周围的碳酸氢根转移到Q B H –的推测机制。
更新日期:2020-11-17
中文翻译:

光诱导的ATR-FTIR光谱揭示了光系统II的铁-醌复合物中关键组氨酸配体的质子化状态
光系统II(PSII)中的铁-醌配合物由两个质体醌电子受体Q A和Q B以及连接它们的非血红素铁组成。有人认为,附近的组氨酸残基在PSII中铁-醌配合物的电子和质子转移反应中起重要作用。在这项研究中,我们使用衰减全反射傅里叶变换红外(ATR-FTIR)光谱技术研究了D1-H215桥接非血红素铁和Q B的质子/去质子反应。闪光诱导的Fe 2+ / Fe 3+ ATR-FTIR差异光谱在5.0-7.5的pH范围内用PSII膜测量。在组氨酸的CN伸展区中,在1094 cm处出现一个负峰强度随着pH值的增加,–1被分配为D1-H215的去质子化阴离子形式。在Fe 3+状态下,奇异值分解分析提供了由于D1-H215的去质子化而ap K a为〜5.5的组分,而在Fe 2+状态下没有组氨酸去质子化的组分。该观察结果支持了先前的建议,即D1-H215负责质子在Fe 2+氧化时的释放[Berthomieu,C.和Hienerwadel,R.(2001)Biochemistry 40,4044–4052]。13的pH依赖性碳酸氢盐配体在非血红素铁上的C同位素编辑谱带进一步显示,在pH <8的Fe 2+或Fe 3+状态下,碳酸氢盐的去质子化反应不会发生。这些结果表明,在整个生理pH范围内,质子通过D1-H215和Fe 2+周围的碳酸氢根转移到Q B H –的推测机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号