当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fractionated regimen-suitable immunoradiotherapy sensitizer based on ultrasmall Fe4Se2W18 nanoclusters enable tumor-specific radiosensitization augment and antitumor immunity boost
Nano Today ( IF 17.4 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.nantod.2020.101003 Ruyi Zhou , Liang Yan , Xinghua Dong , Shuang Zhu , Kui Chen , Yuanzheng Wu , Huandong Xiang , Lele Li , Guangjin Zhang , Zhanjun Gu , Yuliang Zhao
Nano Today ( IF 17.4 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.nantod.2020.101003 Ruyi Zhou , Liang Yan , Xinghua Dong , Shuang Zhu , Kui Chen , Yuanzheng Wu , Huandong Xiang , Lele Li , Guangjin Zhang , Zhanjun Gu , Yuliang Zhao
|
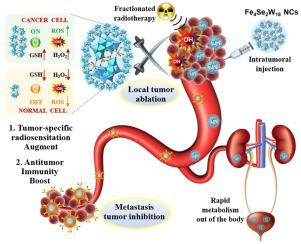
Immunoradiotherapy involving the combination of spatial control of radiotherapy and systemic survival of immunotherapy together has emerged as a promising strategy for both local and systemic tumor rejection. However, immunoradiotherapeutic efficacy is highly impeded by the radiation-induced immunosuppression and the insufficiency of antitumor immunity. Herein, ultrasmall Sandwich-type polyoxotungstate nanoclusters (FeSeW NCs) with abundant high Z elements, efficient catalytic property, and unique electron structure are designed as the immunoradio-sensitizers. Apart from enhancing X-ray deposition for dose reduction, FeSeW NCs exhibit tumor microenvironment-responsive catalytic activity, mainly through GSH depletion and Fenton reaction. Upon X-ray irradiation, FeSeW NCs generate hydroxyl radical cascade to elevate tumor-specific oxidative stress, which can not only selectively ablate the local tumor but also effectively activate antitumor immune response. More importantly, ultrasmall FeSeW NCs can be rapidly eliminated from the body, which can satisfy the needs of the fractionated regimen of radiotherapy clinically to reduce radiation-induced immunosuppression. Immune checkpoint inhibitor (anti-PD-L1 antibody) is further introduced into this system to boost a robust antitumor immunity, resulting in the inhibition of both primary and distant tumors. By presenting the Sandwich-type polyoxotungstate nanoclusters for immunoradiotherapy augmentation, this study is anticipated to establish a novel paradigm for immunoradio-sensitizer design based on polyoxometalate nanoclusters.
中文翻译:

基于超小 Fe4Se2W18 纳米团簇的适合分次治疗方案的免疫放射治疗敏化剂可增强肿瘤特异性放射增敏作用并增强抗肿瘤免疫力
涉及放射治疗的空间控制和免疫治疗的系统生存相结合的免疫放射治疗已成为局部和全身肿瘤排斥的有前途的策略。然而,放射线诱导的免疫抑制和抗肿瘤免疫力的不足严重阻碍了免疫放射治疗的效果。本文设计了具有丰富的高Z元素、高效的催化性能和独特的电子结构的超小三明治型多钨酸纳米团簇(FeSeW NCs)作为免疫放射增敏剂。除了增强X射线沉积以减少剂量外,FeSeW NCs还表现出肿瘤微环境响应性催化活性,主要通过谷胱甘肽消耗和芬顿反应。在X射线照射下,FeSeW NCs产生羟自由基级联反应,升高肿瘤特异性氧化应激,不仅可以选择性消融局部肿瘤,还可以有效激活抗肿瘤免疫反应。更重要的是,超小的FeSeW NCs可以快速从体内消除,可以满足临床上分次放疗方案减少放射引起的免疫抑制的需要。该系统进一步引入免疫检查点抑制剂(抗PD-L1抗体),增强强大的抗肿瘤免疫力,从而抑制原发肿瘤和远处肿瘤。通过提出用于增强免疫放射治疗的三明治型多钨酸盐纳米团簇,本研究预计将为基于多金属氧酸盐纳米团簇的免疫放射增敏剂设计建立新的范例。
更新日期:2020-11-04
中文翻译:

基于超小 Fe4Se2W18 纳米团簇的适合分次治疗方案的免疫放射治疗敏化剂可增强肿瘤特异性放射增敏作用并增强抗肿瘤免疫力
涉及放射治疗的空间控制和免疫治疗的系统生存相结合的免疫放射治疗已成为局部和全身肿瘤排斥的有前途的策略。然而,放射线诱导的免疫抑制和抗肿瘤免疫力的不足严重阻碍了免疫放射治疗的效果。本文设计了具有丰富的高Z元素、高效的催化性能和独特的电子结构的超小三明治型多钨酸纳米团簇(FeSeW NCs)作为免疫放射增敏剂。除了增强X射线沉积以减少剂量外,FeSeW NCs还表现出肿瘤微环境响应性催化活性,主要通过谷胱甘肽消耗和芬顿反应。在X射线照射下,FeSeW NCs产生羟自由基级联反应,升高肿瘤特异性氧化应激,不仅可以选择性消融局部肿瘤,还可以有效激活抗肿瘤免疫反应。更重要的是,超小的FeSeW NCs可以快速从体内消除,可以满足临床上分次放疗方案减少放射引起的免疫抑制的需要。该系统进一步引入免疫检查点抑制剂(抗PD-L1抗体),增强强大的抗肿瘤免疫力,从而抑制原发肿瘤和远处肿瘤。通过提出用于增强免疫放射治疗的三明治型多钨酸盐纳米团簇,本研究预计将为基于多金属氧酸盐纳米团簇的免疫放射增敏剂设计建立新的范例。


























 京公网安备 11010802027423号
京公网安备 11010802027423号