当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Altered copper homeostasis underlies sensitivity of hepatocellular carcinoma to copper chelation
Metallomics ( IF 3.4 ) Pub Date : 2020-11-2 , DOI: 10.1039/d0mt00156b Caroline I Davis 1 , Xingxing Gu , Ryan M Kiefer , Martina Ralle , Terence P Gade , Donita C Brady
Metallomics ( IF 3.4 ) Pub Date : 2020-11-2 , DOI: 10.1039/d0mt00156b Caroline I Davis 1 , Xingxing Gu , Ryan M Kiefer , Martina Ralle , Terence P Gade , Donita C Brady
Affiliation
|
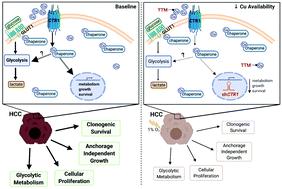
Hepatocellular carcinoma (HCC), the most common primary liver cancer, of which ∼800 000 new cases will be diagnosed worldwide this year, portends a five-year survival rate of merely 17% in patients with unresectable disease. This dismal prognosis is due, at least in part, from the late stage of diagnosis and the limited efficacy of systemic therapies. As a result, there is an urgent need to identify risk factors that contribute to HCC initiation and provide targetable vulnerabilities to improve patient survival. While myriad risk factors are known, elevated copper (Cu) levels in HCC patients and the incidence of hepatobiliary malignancies in Wilson disease patients, which exhibit hereditary liver Cu overload, suggests the possibility that metal accumulation promotes malignant transformation. Here we found that expression of the Cu transporter genes ATP7A, ATP7B, SLC31A1, and SLC31A2 was significantly altered in liver cancer samples and were associated with elevated Cu levels in liver cancer tissue and cells. Further analysis of genomic copy number data revealed that alterations in Cu transporter gene loci correlate with poorer survival in HCC patients. Genetic loss of the Cu importer SLC31A1 (CTR1) or pharmacologic suppression of Cu decreased the viability, clonogenic survival, and anchorage-independent growth of human HCC cell lines. Mechanistically, CTR1 knockdown or Cu chelation decreased glycolytic gene expression and downstream metabolite utilization and as a result forestalled tumor cell survival after exposure to hypoxia, which mimics oxygen deprivation elicited by transarterial embolization, a standard-of-care therapy used for patients with unresectable HCC. Taken together, these findings established an association between altered Cu homeostasis and HCC and suggest that limiting Cu bioavailability may provide a new treatment strategy for HCC by restricting the metabolic reprogramming necessary for cancer cell survival.
中文翻译:

铜稳态的改变是肝细胞癌对铜螯合敏感性的基础
肝细胞癌 (HCC) 是最常见的原发性肝癌,今年全球将诊断出约 80 万例新病例,预示着无法切除的患者的五年生存率仅为 17%。这种惨淡的预后至少部分是由于诊断晚期和全身治疗的疗效有限造成的。因此,迫切需要确定导致 HCC 发生的风险因素,并提供有针对性的脆弱性以提高患者的生存率。虽然已知多种危险因素,但 HCC 患者铜 (Cu) 水平升高以及威尔逊病患者肝胆恶性肿瘤的发病率(表现出遗传性肝脏铜超载)表明金属积累可能会促进恶性转化。在这里,我们发现肝癌样本中铜转运蛋白基因ATP7A、ATP7B、SLC31A1和SLC31A2的表达显着改变,并且与肝癌组织和细胞中铜水平升高相关。对基因组拷贝数数据的进一步分析表明,铜转运蛋白基因位点的改变与 HCC 患者较差的生存率相关。Cu 输入蛋白SLC31A1 ( CTR1 ) 的遗传缺失或 Cu 的药物抑制会降低人 HCC 细胞系的活力、克隆形成存活和贴壁依赖性生长。从机制上讲,CTR1敲低或铜螯合降低了糖酵解基因表达和下游代谢物利用,从而阻止了肿瘤细胞在缺氧后的存活,这类似于经动脉栓塞引起的缺氧,这是一种用于不可切除的肝癌患者的标准治疗方法。总而言之,这些发现建立了铜稳态改变与肝癌之间的关联,并表明限制铜的生物利用度可能通过限制癌细胞生存所需的代谢重编程为肝癌提供新的治疗策略。
更新日期:2021-01-06
中文翻译:

铜稳态的改变是肝细胞癌对铜螯合敏感性的基础
肝细胞癌 (HCC) 是最常见的原发性肝癌,今年全球将诊断出约 80 万例新病例,预示着无法切除的患者的五年生存率仅为 17%。这种惨淡的预后至少部分是由于诊断晚期和全身治疗的疗效有限造成的。因此,迫切需要确定导致 HCC 发生的风险因素,并提供有针对性的脆弱性以提高患者的生存率。虽然已知多种危险因素,但 HCC 患者铜 (Cu) 水平升高以及威尔逊病患者肝胆恶性肿瘤的发病率(表现出遗传性肝脏铜超载)表明金属积累可能会促进恶性转化。在这里,我们发现肝癌样本中铜转运蛋白基因ATP7A、ATP7B、SLC31A1和SLC31A2的表达显着改变,并且与肝癌组织和细胞中铜水平升高相关。对基因组拷贝数数据的进一步分析表明,铜转运蛋白基因位点的改变与 HCC 患者较差的生存率相关。Cu 输入蛋白SLC31A1 ( CTR1 ) 的遗传缺失或 Cu 的药物抑制会降低人 HCC 细胞系的活力、克隆形成存活和贴壁依赖性生长。从机制上讲,CTR1敲低或铜螯合降低了糖酵解基因表达和下游代谢物利用,从而阻止了肿瘤细胞在缺氧后的存活,这类似于经动脉栓塞引起的缺氧,这是一种用于不可切除的肝癌患者的标准治疗方法。总而言之,这些发现建立了铜稳态改变与肝癌之间的关联,并表明限制铜的生物利用度可能通过限制癌细胞生存所需的代谢重编程为肝癌提供新的治疗策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号