当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface functionalized nanoscale metal oxides for arsenic(V), chromium(VI), and uranium(VI) sorption: considering single- and multi-sorbate dynamics
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2020-10-23 , DOI: 10.1039/d0en00728e Changwoo Kim 1, 2, 3, 4 , Seung Soo Lee 4, 5, 6, 7 , Kit Tan Kwan 8, 9, 10, 11 , Junseok Lee 1, 2, 3, 4 , Wenlu Li 1, 2, 3, 4 , Brandon J. Lafferty 4, 12, 13, 14 , Daniel E. Giammar 4, 5, 6, 7 , John D. Fortner 1, 2, 3, 4
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2020-10-23 , DOI: 10.1039/d0en00728e Changwoo Kim 1, 2, 3, 4 , Seung Soo Lee 4, 5, 6, 7 , Kit Tan Kwan 8, 9, 10, 11 , Junseok Lee 1, 2, 3, 4 , Wenlu Li 1, 2, 3, 4 , Brandon J. Lafferty 4, 12, 13, 14 , Daniel E. Giammar 4, 5, 6, 7 , John D. Fortner 1, 2, 3, 4
Affiliation
|
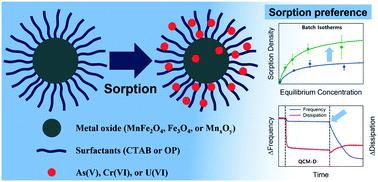
Surface-functionalized Mn–Fe oxide nanocrystals (NCs) were evaluated for single- and multi-sorbate scenarios considering As(V), Cr(VI), and U(VI) in varied water chemistries (deionized (DI), ground, and sea water) at pH 7.0. Multi-sorbate scenarios were further examined for competitive and/or cooperative effects. Precisely synthesized manganese ferrite (MnFe2O4) NCs were compared with iron oxide (Fe3O4) and manganese oxide (MnxOy) nanocrystal cores in terms of sorption capacities and colloidal stabilities. Positively charged cetyltrimethylammonium bromide (CTAB) and negatively charged oleyl phosphate (OP) were evaluated and compared as organic coatings. MnFe2O4 NCs exhibited both enhanced sorption performance and colloidal stability compared with Fe3O4 and MnxOy NC cores when functionalized with the same surfactant coating. For MnFe2O4 NCs, maximum sorption of As(V), Cr(VI), and U(VI) were observed to be 2.62, 3.43, and 4.27 mmol g−1, respectively (in DI water). Relative sorption capacity enhancement (compared with Fe3O4 and MnxOy) is due to increased surface grafting densities of MnFe2O4 NCs, providing a larger number of sorption sites (functional group) for target sorbates and higher repulsive energy (osmotic and elastic–steric interaction) for increased stability, and thus maintaining available surface area. For As(V) and Cr(VI) multi-sorbate systems, all materials evaluated preferentially adsorbed As(V) over Cr(VI). This preference was further investigated and observed using a novel quartz crystal microbalance (QCM) technique.
中文翻译:

用于砷(V),铬(VI)和铀(VI)吸附的表面功能化纳米金属氧化物:考虑单和多吸附物动力学
考虑到在各种水化学(去离子(DI),地面和水中)中的As(V),Cr(VI)和U(VI),对表面官能化的Mn-Fe氧化物纳米晶体(NCs)进行了单吸附和多吸附情况的评估。海水)pH 7.0。进一步研究了多山梨酸酯的竞争和/或合作效果。将精确合成的锰铁氧体(MnFe 2 O 4)NCs与氧化铁(Fe 3 O 4)和氧化锰(Mn x O y纳米晶核的吸附能力和胶体稳定性。对带正电的十六烷基三甲基溴化铵(CTAB)和带负电的油基磷酸酯(OP)进行了评估,并将其作为有机涂料进行比较。与相同表面活性剂涂层功能化的Fe 3 O 4和Mn x O y NC核相比,MnFe 2 O 4 NCs表现出增强的吸附性能和胶体稳定性。对于MnFe 2 O 4 NCs,观察到As(V),Cr(VI)和U(VI)的最大吸附为2.62、3.43和4.27 mmol g -1,分别(在去离子水中)。相对吸附能力的提高(与Fe 3 O 4和Mn x O y相比)是由于MnFe 2 O 4 NCs的表面接枝密度增加,从而为目标被吸附物提供了更多的吸附位点(官能团)和更高的排斥能(渗透和弹性-空间相互作用),以增加稳定性,从而保持可用的表面积。对于As(V)和Cr(VI)多山梨醇酯体系,所有评估的材料均优先于Cr(VI)吸附As(V))。使用新型石英晶体微量天平(QCM)技术进一步研究和观察了这种偏好。
更新日期:2020-11-04
中文翻译:

用于砷(V),铬(VI)和铀(VI)吸附的表面功能化纳米金属氧化物:考虑单和多吸附物动力学
考虑到在各种水化学(去离子(DI),地面和水中)中的As(V),Cr(VI)和U(VI),对表面官能化的Mn-Fe氧化物纳米晶体(NCs)进行了单吸附和多吸附情况的评估。海水)pH 7.0。进一步研究了多山梨酸酯的竞争和/或合作效果。将精确合成的锰铁氧体(MnFe 2 O 4)NCs与氧化铁(Fe 3 O 4)和氧化锰(Mn x O y纳米晶核的吸附能力和胶体稳定性。对带正电的十六烷基三甲基溴化铵(CTAB)和带负电的油基磷酸酯(OP)进行了评估,并将其作为有机涂料进行比较。与相同表面活性剂涂层功能化的Fe 3 O 4和Mn x O y NC核相比,MnFe 2 O 4 NCs表现出增强的吸附性能和胶体稳定性。对于MnFe 2 O 4 NCs,观察到As(V),Cr(VI)和U(VI)的最大吸附为2.62、3.43和4.27 mmol g -1,分别(在去离子水中)。相对吸附能力的提高(与Fe 3 O 4和Mn x O y相比)是由于MnFe 2 O 4 NCs的表面接枝密度增加,从而为目标被吸附物提供了更多的吸附位点(官能团)和更高的排斥能(渗透和弹性-空间相互作用),以增加稳定性,从而保持可用的表面积。对于As(V)和Cr(VI)多山梨醇酯体系,所有评估的材料均优先于Cr(VI)吸附As(V))。使用新型石英晶体微量天平(QCM)技术进一步研究和观察了这种偏好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号