Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bafilomycin A1 enhances NLRP3 inflammasome activation in human monocytes independent of lysosomal acidification
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-11-03 , DOI: 10.1111/febs.15619 Shi Yu 1, 2 , Jack Green 1, 2 , Rose Wellens 1, 2 , Gloria Lopez-Castejon 2, 3 , David Brough 1, 2
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-11-03 , DOI: 10.1111/febs.15619 Shi Yu 1, 2 , Jack Green 1, 2 , Rose Wellens 1, 2 , Gloria Lopez-Castejon 2, 3 , David Brough 1, 2
Affiliation
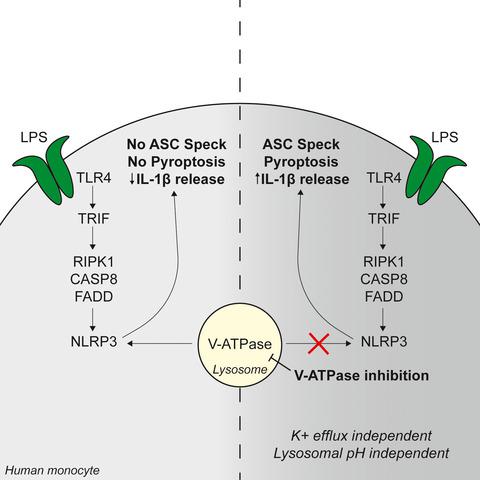
|
The release of interleukin (IL)‐1β from primary human monocytes in response to extracellular LPS occurs through the NACHT, LRR and PYD domains‐containing protein 3 (NLRP3) inflammasome. In primary monocytes, in response to LPS, NLRP3 inflammasome activation is characterized by an independence of K+ efflux and ASC speck formation and has been termed the ‘alternative’ pathway. Here, we report that pharmacological inhibition of V‐ATPase with bafilomycin A1 exacerbated LPS‐induced NLRP3 inflammasome activation in primary human monocytes. Inhibition of V‐ATPase in the presence of extracellular LPS led to NLRP3‐dependent, K+ efflux‐independent, ASC oligomerization and caspase‐1 activation. Although V‐ATPases are required for lysosomal acidification, we found that acidic lysosomal pH and protease activity were dispensable for this altered response, suggesting that V‐ATPase inhibition triggered alternative signalling events. Therefore, V‐ATPases may serve additional roles during NLRP3 inflammasome activation in primary human monocytes.
中文翻译:

Bafilomycin A1 增强人单核细胞中 NLRP3 炎症小体的激活,与溶酶体酸化无关
白细胞介素 (IL)-1β 从原代人单核细胞中释放以响应细胞外 LPS,是通过含有 NACHT、LRR 和 PYD 结构域的蛋白 3 (NLRP3) 炎性体发生的。在原代单核细胞中,响应 LPS,NLRP3 炎性体激活的特征在于 K +流出和 ASC 斑点形成的独立性,并被称为“替代”途径。在这里,我们报告了巴弗洛霉素 A1 对 V-ATP 酶的药理学抑制加剧了 LPS 诱导的原代人单核细胞中 NLRP3 炎性体的活化。在细胞外 LPS 存在下抑制 V-ATPase 导致 NLRP3 依赖性、K +不依赖外排、ASC 寡聚化和 caspase-1 激活。尽管溶酶体酸化需要 V-ATP 酶,但我们发现酸性溶酶体 pH 值和蛋白酶活性对于这种改变的反应是可有可无的,这表明 V-ATP 酶抑制触发了替代信号事件。因此,V-ATPases 可能在人原代单核细胞的 NLRP3 炎性体激活过程中发挥额外的作用。
更新日期:2020-11-03
中文翻译:

Bafilomycin A1 增强人单核细胞中 NLRP3 炎症小体的激活,与溶酶体酸化无关
白细胞介素 (IL)-1β 从原代人单核细胞中释放以响应细胞外 LPS,是通过含有 NACHT、LRR 和 PYD 结构域的蛋白 3 (NLRP3) 炎性体发生的。在原代单核细胞中,响应 LPS,NLRP3 炎性体激活的特征在于 K +流出和 ASC 斑点形成的独立性,并被称为“替代”途径。在这里,我们报告了巴弗洛霉素 A1 对 V-ATP 酶的药理学抑制加剧了 LPS 诱导的原代人单核细胞中 NLRP3 炎性体的活化。在细胞外 LPS 存在下抑制 V-ATPase 导致 NLRP3 依赖性、K +不依赖外排、ASC 寡聚化和 caspase-1 激活。尽管溶酶体酸化需要 V-ATP 酶,但我们发现酸性溶酶体 pH 值和蛋白酶活性对于这种改变的反应是可有可无的,这表明 V-ATP 酶抑制触发了替代信号事件。因此,V-ATPases 可能在人原代单核细胞的 NLRP3 炎性体激活过程中发挥额外的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号