Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.jmb.2020.10.031 Yanan He 1 , Pragati Agnihotri 2 , Sneha Rangarajan 2 , Yihong Chen 1 , Melissa C Kerzic 2 , Buyong Ma 3 , Ruth Nussinov 4 , Roy A Mariuzza 2 , John Orban 1
|
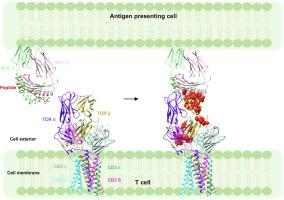
T cells are vital for adaptive immune responses that protect against pathogens and cancers. The T cell receptor (TCR)–CD3 complex comprises a diverse αβ TCR heterodimer in noncovalent association with three invariant CD3 dimers. The TCR is responsible for recognizing antigenic peptides bound to MHC molecules (pMHC), while the CD3 dimers relay activation signals to the T cell. However, the mechanisms by which TCR engagement by pMHC is transmitted to CD3 remain mysterious, although there is growing evidence that mechanosensing and allostery both play a role. Here, we carried out NMR analysis of a human autoimmune TCR (MS2-3C8) that recognizes a self-peptide from myelin basic protein presented by the MHC class II molecule HLA-DR4. We observed pMHC-induced NMR signal perturbations in MS2-3C8 that indicate long-range effects on TCR β chain conformation and dynamics. Our results demonstrate that, in addition to expected changes in the NMR resonances of pMHC-contacting residues, perturbations extend to the Vβ/Vα, Vβ/Cβ, and Cβ/Cα interfacial regions. Moreover, the pattern of long-range perturbations is similar to that detected previously in the β chains of two MHC class I-restricted TCRs, thereby revealing a common allosteric pathway among three unrelated TCRs. Molecular dynamics (MD) simulations predict similar pMHC-induced effects. Taken together, our results demonstrate that pMHC binding induces long-range allosteric changes in the TCR β chain at conserved sites in both representative MHC class I- and class II-restricted TCRs, and that these sites may play a role in the transmission of signaling information.
中文翻译:

肽-MHC 结合揭示了 MHC I 类和 II 类限制性 T 细胞受体 (TCR) 中的保守变构位点
T 细胞对于抵御病原体和癌症的适应性免疫反应至关重要。T 细胞受体 (TCR)-CD3 复合物包含与三个不变的 CD3 二聚体非共价结合的多样化 αβ TCR 异源二聚体。TCR 负责识别与 MHC 分子 (pMHC) 结合的抗原肽,而 CD3 二聚体将激活信号传递给 T 细胞。然而,尽管越来越多的证据表明机械感应和变构都起作用,但 pMHC 将 TCR 参与传递到 CD3 的机制仍然是个谜。在这里,我们对人类自身免疫性 TCR (MS2-3C8) 进行了 NMR 分析,该 TCR 识别来自 MHC II 类分子 HLA-DR4 呈递的髓鞘碱性蛋白的自身肽。我们在 MS2-3C8 中观察到 pMHC 诱导的 NMR 信号扰动,表明对 TCR β 链构象和动力学的长期影响。我们的结果表明,除了 pMHC 接触残基的 NMR 共振预期变化之外,扰动还扩展到 Vβ/Vα、Vβ/Cβ 和 Cβ/Cα 界面区域。此外,远程扰动的模式类似于先前在两个 MHC I 类限制性 TCR 的 β 链中检测到的模式,从而揭示了三个不相关的 TCR 之间的共同变构途径。分子动力学 (MD) 模拟预测类似的 pMHC 诱导效应。总之,我们的结果表明 pMHC 结合在具有代表性的 MHC I 类和 II 类限制性 TCR 的保守位点处诱导 TCR β 链的长程变构变化,


























 京公网安备 11010802027423号
京公网安备 11010802027423号