当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic and gas-phase study of the chemical vapor deposition of silicon carbide from C2H3SiCl3/H2
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jiec.2020.10.029 A. Desenfant , G. Laduye , G.L. Vignoles , G. Chollon
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jiec.2020.10.029 A. Desenfant , G. Laduye , G.L. Vignoles , G. Chollon
|
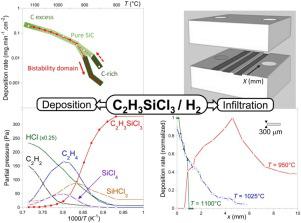
Abstract The chemical vapor deposition (CVD) of silicon carbide from vinyltrichlorosilane (VTS) was studied to identify a range of conditions leading to pure crystalline SiC. The deposition rate was recorded to evidence the various deposition regimes. Gas phase, elemental analyses and infiltration tests were also performed. Three distinct chemical reaction regimes were identified. In CVD conditions, carbon is co-deposited at low temperature while VTS is only partially decomposed. In infiltration conditions, the composition switches to pure SiC inside the porous substrate because of a depletion of reactive hydrocarbon species. Competing heterogeneous reactions are responsible for a hysteresis versus temperature, in both deposition rate and composition of the deposit. The high temperature domain is the most suitable to deposit pure crystalline SiC in CVD conditions. Hydrogen dilution strongly accelerates the homogeneous decomposition of VTS as compared to argon. Assumptions on the reaction mechanism were proposed describing the chemistry of this precursor.
中文翻译:

从 C2H3SiCl3/H2 化学气相沉积碳化硅的动力学和气相研究
摘要 对来自乙烯基三氯硅烷 (VTS) 的碳化硅的化学气相沉积 (CVD) 进行了研究,以确定导致纯结晶 SiC 的一系列条件。记录沉积速率以证明各种沉积方式。还进行了气相、元素分析和渗透测试。确定了三种不同的化学反应方式。在 CVD 条件下,碳在低温下共沉积,而 VTS 仅部分分解。在渗透条件下,由于活性烃类物质的消耗,该组合物在多孔基材内转变为纯 SiC。在沉积速率和沉积物组成方面,相互竞争的非均相反应是造成温度滞后的原因。高温域最适合在 CVD 条件下沉积纯晶体 SiC。与氩气相比,氢气稀释强烈加速了 VTS 的均匀分解。提出了对反应机理的假设来描述该前体的化学性质。
更新日期:2021-02-01
中文翻译:

从 C2H3SiCl3/H2 化学气相沉积碳化硅的动力学和气相研究
摘要 对来自乙烯基三氯硅烷 (VTS) 的碳化硅的化学气相沉积 (CVD) 进行了研究,以确定导致纯结晶 SiC 的一系列条件。记录沉积速率以证明各种沉积方式。还进行了气相、元素分析和渗透测试。确定了三种不同的化学反应方式。在 CVD 条件下,碳在低温下共沉积,而 VTS 仅部分分解。在渗透条件下,由于活性烃类物质的消耗,该组合物在多孔基材内转变为纯 SiC。在沉积速率和沉积物组成方面,相互竞争的非均相反应是造成温度滞后的原因。高温域最适合在 CVD 条件下沉积纯晶体 SiC。与氩气相比,氢气稀释强烈加速了 VTS 的均匀分解。提出了对反应机理的假设来描述该前体的化学性质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号