Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.bbamem.2020.183504 Stéphane Abel , Massimo Marchi , Justine Solier , Stéphanie Finet , Karl Brillet , Françoise Bonneté
|
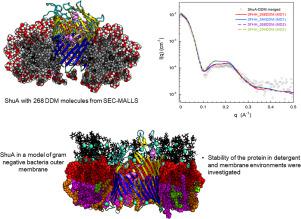
Successful crystallization of membrane proteins in detergent micelles depends on key factors such as conformational stability of the protein in micellar assemblies, the protein-detergent complex (PDC) monodispersity and favorable protein crystal contacts by suitable shielding of the protein hydrophobic surface by the detergent belt. With the aim of studying the influence of amphiphilic environment on membrane protein structure, stability and crystallizability, we combine molecular dynamics (MD) simulations with SEC-MALLS and SEC-SAXS (Size Exclusion Chromatography in line with Multi Angle Laser Light Scattering or Small Angle X-ray Scattering) experiments to describe the protein-detergent interactions that could help to rationalize PDC crystallization. In this context, we compare the protein-detergent interactions of ShuA from Shigella dysenteriae in n-Dodecyl-β-D-Maltopyranoside (DDM) with ShuA inserted in a realistic model of gram-negative bacteria outer membrane (OM) containing a mixture of bacterial lipopolysaccharide and phospholipids. To evaluate the quality of the PDC models, we compute the corresponding SAXS curves from the MD trajectories and compare with the experimental ones. We show that computed SAXS curves obtained from the MD trajectories reproduce better the SAXS obtained from the SEC-SAXS experiments for ShuA surrounded by 268 DDM molecules. The MD results show that the DDM molecules form around ShuA a closed belt whose the hydrophobic thickness appears slightly smaller (~22 Å) than the hydrophobic transmembrane domain of the protein (24.6 Å) suggested by Orientations of Proteins in Membranes (OPM) database. The simulations also show that ShuA transmembrane domain is remarkably stable in all the systems except for the extracellular and periplasmic loops that exhibit larger movements due to specific molecular interactions with lipopolysaccharides (LPS). We finally point out that this detergent behavior may lead to the occlusion of the periplasmic hydrophilic surface and poor crystal contacts leading to difficulties in crystallization of ShuA in DDM.
中文翻译:

通过SAXS和MD模拟可以看到DDM胶束和革兰氏阴性细菌外膜模型中膜受体ShuA的结构洞察力
洗涤剂胶束中膜蛋白的成功结晶取决于关键因素,例如胶束装配体中蛋白的构象稳定性,蛋白洗涤剂复合物(PDC)的单分散性以及通过洗涤剂带对蛋白疏水表面的适当屏蔽,有利的蛋白晶体接触。为了研究两亲环境对膜蛋白结构,稳定性和结晶性的影响,我们将分子动力学(MD)模拟与SEC-MALLS和SEC-SAXS(尺寸排阻色谱法结合多角度激光散射或小角度分析)相结合X射线散射)实验来描述可以帮助合理化PDC结晶的蛋白质洗涤剂相互作用。在这种情况下,我们比较了ShuA的蛋白质-洗涤剂相互作用痢疾志贺氏菌将ShuA插入含有细菌脂多糖和磷脂混合物的革兰氏阴性细菌外膜(OM)的真实模型中的ShuA插入正十二烷基-β-D-麦芽吡喃糖苷(DDM)中。为了评估PDC模型的质量,我们从MD轨迹计算出相应的SAXS曲线,并与实验曲线进行比较。我们表明,从MD轨迹获得的计算SAXS曲线可更好地再现从268个DDM分子包围的ShuA的SEC-SAXS实验获得的SAXS。MD结果表明,DDM分子在ShuA周围形成一条封闭的带,其疏水性厚度似乎比膜的疏水性跨膜结构域(24.6Å)略小(〜22Å),这是膜中蛋白质的方向(OPM)数据库建议的。模拟还显示,ShuA跨膜结构域在所有系统中均非常稳定,但由于与脂多糖(LPS)的特定分子相互作用,胞外和周质环表现出较大的运动。我们最终指出,这种去污剂的行为可能会导致周质亲水表面的堵塞和不良的晶体接触,从而导致DDM中ShuA的结晶困难。



























 京公网安备 11010802027423号
京公网安备 11010802027423号