Results in Chemistry Pub Date : 2020-08-19 , DOI: 10.1016/j.rechem.2020.100067 B.J. Ullas , K.P. Rakesh , J. Shivakumar , D. Channe Gowda , P.G. Chandrashekara
|
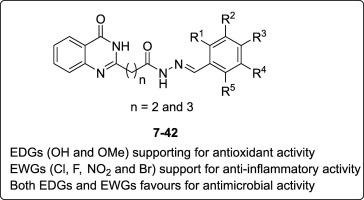
Two series of quinazolinone derived Schiff's bases were synthesized and characterized by analytical and spectroscopic techniques. All the compounds were screened for their in vitro biological studies. The in vitro antioxidant activities of these compounds were evaluated employing 1,1-diphenyl-2-picryl-hydrazyl (DPPH), 2,2-azinobis-(3-ethylbenzothiazoline-6-sufonic acid) (ABTS+) and N,N-dimethyl-p-phenylenediamine dihydrochloride (DMPD+) radical assay. The results revealed that compounds 31 (IC50: 35 μg/mL) and 32 (IC50: 40 μg/mL) showed excellent antioxidant activity and their IC50 is lower than the IC50 of standards such as butylated hydroxyl anisole, ascorbic acid and gallic acid in all the three performed assays. In anti-inflammatory activity, compounds 7–26 (IC50: 10-35 μg/mL) showed extraordinary activity compared to standards indomethacin (IC50: 40 μg/mL) and ibuprofen (IC50: 65 μg/mL). In antimicrobial activity, compounds 25–42 shows potent antibacterial activity and compounds 25–42 shows potent antifungal activity compared to standards streptomycin (ZoI: 10 mm) and bavistin (ZoI:10 mm) respectively. To study the structure activity relationship (SAR), electron donating groups (-OH and -OCH3) favors the antioxidant activity, electron withdrawing groups ( F,
F,  Cl,
Cl,  Br and -NO2) favors the anti-inflammatory activity, and both electron donating (-OH and -OCH3) and electron withdrawing (
Br and -NO2) favors the anti-inflammatory activity, and both electron donating (-OH and -OCH3) and electron withdrawing ( F,
F,  Cl,
Cl,  Br and -NO2) groups (25–32) or heterocyclic moiety (33–42) favors antimicrobial activity.
Br and -NO2) groups (25–32) or heterocyclic moiety (33–42) favors antimicrobial activity.
中文翻译:

多靶点喹唑啉酮-席夫氏碱作为有效的生物疗法
合成了两个系列的喹唑啉酮衍生的席夫碱,并通过分析和光谱技术对其进行了表征。筛选所有化合物的体外生物学研究。在体外,这些化合物的抗氧化活性进行了评价采用1,1-二苯基-2-苦基-肼(DPPH),2,2- azinobis-(3-乙基-6- sufonic磺酸)(ABTS +)和Ñ,Ñ -二甲基-对苯二胺盐酸盐(DMPD +)自由基测定。结果显示化合物31(IC 50:35μg/ mL)和32(IC 50:40μg/ mL)表现出优异的抗氧化活性,并且在所有三个执行的测定中,其IC 50均低于丁基化羟基茴香醚,抗坏血酸和没食子酸等标准品的IC 50。与标准消炎痛(IC 50:40μg/ mL)和布洛芬(IC 50:65μg/ mL)相比,化合物7–26(IC 50:10-35μg/ mL)显示出非凡的活性。在抗微生物活性方面,化合物25-42显示有效的抗菌活性,化合物25-42与标准链霉素(ZoI:10 mm)和bavistin(ZoI:10 mm)相比,它们分别显示出强大的抗真菌活性。为了研究结构活性关系(SAR),给电子基团(-OH和-OCH 3)有助于抗氧化活性,吸电子基团( F,
F, Cl,
Cl, Br和-NO 2)具有抗炎活性,并且两个电子给予(-OH和-OCH 3)和吸电子(
Br和-NO 2)具有抗炎活性,并且两个电子给予(-OH和-OCH 3)和吸电子( F,
F, Cl,
Cl, Br和-NO 2)(25-32)或杂环部分(33-42)有利于抗菌活性。
Br和-NO 2)(25-32)或杂环部分(33-42)有利于抗菌活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号