Journal of Structural Biology ( IF 3 ) Pub Date : 2020-11-03 , DOI: 10.1016/j.jsb.2020.107638 Sushant Sadotra , Yuan-Chao Lou , Hao-Cheng Tang , Yi-Chih Chiu , Chun-Hua Hsu , Chinpan Chen
|
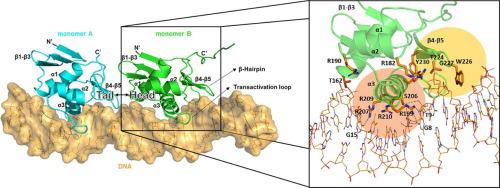
OmpR, a response regulator of the EnvZ/OmpR two-component system (TCS), controls the reciprocal regulation of two porin proteins, OmpF and OmpC, in bacteria. During signal transduction, OmpR (OmpR-FL) undergoes phosphorylation at its conserved Asp residue in the N-terminal receiver domain (OmpRn) and recognizes the promoter DNA from its C-terminal DNA-binding domain (OmpRc) to elicit an adaptive response. Apart from that, OmpR regulates many genes in Escherichia coli and is important for virulence in several pathogens. However, the molecular mechanism of the regulation and the structural basis of OmpR–DNA binding is still not fully clear. In this study, we presented the crystal structure of OmpRc in complex with the F1 region of the ompF promoter DNA from E. coli. Our structural analysis suggested that OmpRc binds to its cognate DNA as a homodimer, only in a head-to-tail orientation. Also, the OmpRc apo-form showed a unique domain-swapped crystal structure under different crystallization conditions. Biophysical experimental data, such as NMR, fluorescent polarization and thermal stability, showed that inactive OmpR-FL (unphosphorylated) could bind to promoter DNA with a weaker binding affinity as compared with active OmpR-FL (phosphorylated) or OmpRc, and also confirmed that phosphorylation may only enhance DNA binding. Furthermore, the dimerization interfaces in the OmpRc–DNA complex structure identified in this study provide an opportunity to understand the regulatory role of OmpR and explore the potential for this “druggable” target.
中文翻译:

响应调节器 OmpR 识别启动子 DNA 的结构基础
OmpR 是 EnvZ/OmpR 双组分系统 (TCS) 的响应调节器,控制细菌中两种孔蛋白 OmpF 和 OmpC 的相互调节。在信号转导过程中,OmpR (OmpR-FL) 在其 N 端接收域 (OmpRn) 中保守的 Asp 残基处经历磷酸化,并识别来自其 C 端 DNA 结合域 (OmpRc) 的启动子 DNA 以引发适应性反应。除此之外,OmpR 调节大肠杆菌中的许多基因,并且对几种病原体的毒力很重要。然而,调控的分子机制和 OmpR-DNA 结合的结构基础仍不完全清楚。在这项研究中,我们展示了 OmpRc 的晶体结构与来自大肠杆菌的ompF启动子 DNA的 F1 区复合. 我们的结构分析表明,OmpRc 与其同源 DNA 结合为同源二聚体,仅以头对尾的方向结合。此外,OmpRc apo 形式在不同的结晶条件下显示出独特的域交换晶体结构。核磁共振、荧光偏振和热稳定性等生物物理实验数据表明,与活性 OmpR-FL(磷酸化)或 OmpRc 相比,非活性 OmpR-FL(未磷酸化)与启动子 DNA 的结合亲和力较弱,同时也证实了磷酸化可能只会增强 DNA 结合。此外,本研究中鉴定的 OmpRc-DNA 复合结构中的二聚化界面为了解 OmpR 的调节作用和探索这一“可成药”目标的潜力提供了机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号