Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modeling ascending infection with a feto-maternal interface organ-on-chip
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-10-28 , DOI: 10.1039/d0lc00875c Lauren S Richardson 1 , Sungjin Kim , Arum Han , Ramkumar Menon
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-10-28 , DOI: 10.1039/d0lc00875c Lauren S Richardson 1 , Sungjin Kim , Arum Han , Ramkumar Menon
Affiliation
|
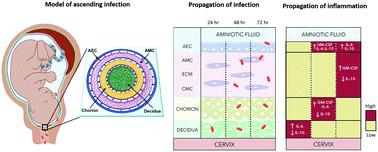
Maternal infection (i.e., ascending infection) and the resulting host inflammatory response are risk factors associated with spontaneous preterm birth (PTB), a major pregnancy complication. However, the path of infection and its propagation from the maternal side to the fetal side have been difficult to study due to the lack of appropriate in vitro models and limitations of animal models. A better understanding of the propagation kinetics of infectious agents and development of the host inflammatory response at the feto-maternal (amniochorion-decidua, respectively) interface (FMi) is critical in curtailing host inflammatory responses that can lead to PTB. To model ascending infection and determine inflammatory responses at the FMi, we developed a microfluidic organ-on-chip (OOC) device containing primary cells from the FMi (decidua, chorion, and amnion [mesenchyme and epithelium]) and collagen matrix harvested from primary tissue. The FMi-OOC is composed of four concentric circular cell/collagen chambers designed to mimic the thickness and cell density of the FMi in vivo. Each layer is connected by arrays of microchannels filled with type IV collagen to recreate the basement membrane of the amniochorion. Cellular characteristics (viability, morphology, production of nascent collagen, cellular transitions, and migration) in the OOC were similar to those seen in utero, validating the physiological relevance and utility of the developed FMi-OOC. The ascending infection model of the FMi-OOC, triggered by exposing the maternal (decidua) side of the OOC to lipopolysaccharide (LPS, 100 ng mL−1), shows that LPS propagated through the chorion, amnion mesenchyme, and reached the fetal amnion within 72 h. LPS induced time-dependent and cell-type-specific pro-inflammatory cytokine production (24 h decidua: IL-6, 48 h chorion: GM-CSF and IL-6, and 72 h amnion mesenchyme and epithelium: GM-CSF and IL-6). Collectively, this OOC model and study successfully modeled ascending infection, its propagation, and distinct inflammatory response at the FMi indicative of pathologic pathways of PTB. This OOC model provides a novel platform to study physiological and pathological cell status at the FMi, and is expected to have broad utility in the field of obstetrics.
中文翻译:

使用胎儿-母体界面器官芯片模拟上行感染
母体感染(即上行感染)和由此产生的宿主炎症反应是与自发性早产(PTB)(一种主要妊娠并发症)相关的危险因素。然而,由于缺乏合适的体外模型和动物模型的局限性,感染途径及其从母体到胎儿的传播途径一直难以研究。更好地了解传染源的传播动力学以及胎儿-母体(羊膜-蜕膜)界面(FMi)宿主炎症反应的发展对于减少可导致 PTB 的宿主炎症反应至关重要。为了模拟上行感染并确定 FMi 的炎症反应,我们开发了一种微流控芯片器官 (OOC) 装置,其中包含来自 FMi 的原代细胞(蜕膜、绒毛膜和羊膜 [间质和上皮])和从原代细胞收获的胶原基质组织。FMi-OOC 由四个同心圆形细胞/胶原室组成,旨在模拟体内FMi 的厚度和细胞密度。每层都通过充满 IV 型胶原蛋白的微通道阵列连接,以重建羊膜的基底膜。OOC 中的细胞特征(活力、形态、新生胶原蛋白的产生、细胞转变和迁移)与子宫内观察到的细胞特征相似,验证了所开发的 FMi-OOC 的生理相关性和实用性。FMi-OOC 的上行感染模型是通过将 OOC 的母体(蜕膜)侧暴露于脂多糖(LPS,100 ng mL -1)而触发的,表明 LPS 通过绒毛膜、羊膜间质传播并到达胎儿羊膜72 小时内。LPS 诱导时间依赖性和细胞类型特异性促炎细胞因子的产生(24 小时蜕膜:IL-6,48 小时绒毛膜:GM-CSF 和 IL-6,72 小时羊膜间质和上皮:GM-CSF 和 IL -6)。总的来说,这个 OOC 模型和研究成功地模拟了上行感染、其传播以及指示 PTB 病理途径的 FMi 处的独特炎症反应。该 OOC 模型为研究 FMi 的生理和病理细胞状态提供了一个新的平台,预计在产科领域具有广泛的实用性。
更新日期:2020-11-03
中文翻译:

使用胎儿-母体界面器官芯片模拟上行感染
母体感染(即上行感染)和由此产生的宿主炎症反应是与自发性早产(PTB)(一种主要妊娠并发症)相关的危险因素。然而,由于缺乏合适的体外模型和动物模型的局限性,感染途径及其从母体到胎儿的传播途径一直难以研究。更好地了解传染源的传播动力学以及胎儿-母体(羊膜-蜕膜)界面(FMi)宿主炎症反应的发展对于减少可导致 PTB 的宿主炎症反应至关重要。为了模拟上行感染并确定 FMi 的炎症反应,我们开发了一种微流控芯片器官 (OOC) 装置,其中包含来自 FMi 的原代细胞(蜕膜、绒毛膜和羊膜 [间质和上皮])和从原代细胞收获的胶原基质组织。FMi-OOC 由四个同心圆形细胞/胶原室组成,旨在模拟体内FMi 的厚度和细胞密度。每层都通过充满 IV 型胶原蛋白的微通道阵列连接,以重建羊膜的基底膜。OOC 中的细胞特征(活力、形态、新生胶原蛋白的产生、细胞转变和迁移)与子宫内观察到的细胞特征相似,验证了所开发的 FMi-OOC 的生理相关性和实用性。FMi-OOC 的上行感染模型是通过将 OOC 的母体(蜕膜)侧暴露于脂多糖(LPS,100 ng mL -1)而触发的,表明 LPS 通过绒毛膜、羊膜间质传播并到达胎儿羊膜72 小时内。LPS 诱导时间依赖性和细胞类型特异性促炎细胞因子的产生(24 小时蜕膜:IL-6,48 小时绒毛膜:GM-CSF 和 IL-6,72 小时羊膜间质和上皮:GM-CSF 和 IL -6)。总的来说,这个 OOC 模型和研究成功地模拟了上行感染、其传播以及指示 PTB 病理途径的 FMi 处的独特炎症反应。该 OOC 模型为研究 FMi 的生理和病理细胞状态提供了一个新的平台,预计在产科领域具有广泛的实用性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号