当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
What Are We Missing by Using Hydrophilic Enrichment? Improving Bacterial Glycoproteome Coverage Using Total Proteome and FAIMS Analyses
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-10-30 , DOI: 10.1021/acs.jproteome.0c00565 Ameera Raudah Ahmad Izaham 1 , Ching-Seng Ang 2 , Shuai Nie 2 , Lauren E. Bird 1 , Nicholas A. Williamson 2 , Nichollas E. Scott 1
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-10-30 , DOI: 10.1021/acs.jproteome.0c00565 Ameera Raudah Ahmad Izaham 1 , Ching-Seng Ang 2 , Shuai Nie 2 , Lauren E. Bird 1 , Nicholas A. Williamson 2 , Nichollas E. Scott 1
Affiliation
|
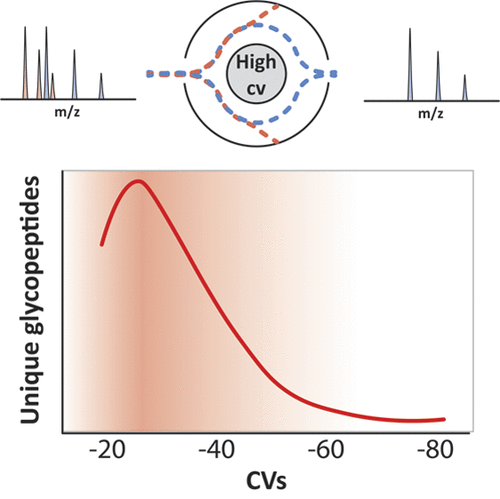
Hydrophilic interaction liquid chromatography (HILIC) glycopeptide enrichment is an indispensable tool for the high-throughput characterization of glycoproteomes. Despite its utility, HILIC enrichment is associated with a number of shortcomings, including requiring large amounts of starting materials, potentially introducing chemical artifacts such as formylation when high concentrations of formic acid are used, and biasing/undersampling specific classes of glycopeptides. Here, we investigate HILIC enrichment-independent approaches for the study of bacterial glycoproteomes. Using three Burkholderia species (Burkholderia cenocepacia, Burkholderia Dolosa, and Burkholderia ubonensis), we demonstrate that short aliphatic O-linked glycopeptides are typically absent from HILIC enrichments, yet are readily identified in whole proteome samples. Using high-field asymmetric waveform ion mobility spectrometry (FAIMS) fractionation, we show that at high compensation voltages (CVs), short aliphatic glycopeptides can be enriched from complex samples, providing an alternative means to identify glycopeptide recalcitrant to hydrophilic-based enrichment. Combining whole proteome and FAIMS analyses, we show that the observable glycoproteome of these Burkholderia species is at least 25% larger than what was initially thought. Excitingly, the ability to enrich glycopeptides using FAIMS appears generally applicable, with the N-linked glycopeptides of Campylobacter fetus subsp. fetus also being enrichable at high FAIMS CVs. Taken together, these results demonstrate that FAIMS provides an alternative means to access glycopeptides and is a valuable tool for glycoproteomic analysis.
中文翻译:

使用亲水性浓缩我们缺少了什么?使用总蛋白质组和FAIMS分析提高细菌糖蛋白覆盖率
亲水相互作用液相色谱(HILIC)糖肽富集是糖蛋白组蛋白高通量表征的必不可少的工具。尽管它具有实用性,但HILIC富集还是有许多缺点,包括需要大量的起始原料,当使用高浓度的甲酸时可能会引入化学伪影,例如甲酰化,以及偏向/欠采样特定类别的糖肽。在这里,我们研究细菌糖蛋白组学的HILIC富集独立方法。采用三个伯克霍尔德物种(新洋葱伯克霍尔德菌,鼻疽Dolosa,和鼻疽ubonensis),我们证明HILIC富集通常不存在短的脂族O-连接糖肽,但很容易在整个蛋白质组样品中鉴定出来。使用高场非对称波形离子迁移谱(FAIMS)分离,我们显示出在高补偿电压(CVs)下,短脂族糖肽可以从复杂样品中富集,为鉴定基于亲水性富集的糖肽提供了另一种手段。结合整个蛋白质组和FAIMS分析,我们表明这些伯克霍尔德氏菌物种的可观察到的糖蛋白质组至少比最初认为的大25%。令人兴奋的是,使用FAIMS富集糖肽的能力似乎与弯曲杆菌胎儿亚种的N-连接糖肽普遍适用。胎儿也可以在高FAIMS CV条件下富集。综上所述,这些结果表明,FAIMS提供了一种访问糖肽的替代方法,并且是糖蛋白组学分析的宝贵工具。
更新日期:2021-01-01
中文翻译:

使用亲水性浓缩我们缺少了什么?使用总蛋白质组和FAIMS分析提高细菌糖蛋白覆盖率
亲水相互作用液相色谱(HILIC)糖肽富集是糖蛋白组蛋白高通量表征的必不可少的工具。尽管它具有实用性,但HILIC富集还是有许多缺点,包括需要大量的起始原料,当使用高浓度的甲酸时可能会引入化学伪影,例如甲酰化,以及偏向/欠采样特定类别的糖肽。在这里,我们研究细菌糖蛋白组学的HILIC富集独立方法。采用三个伯克霍尔德物种(新洋葱伯克霍尔德菌,鼻疽Dolosa,和鼻疽ubonensis),我们证明HILIC富集通常不存在短的脂族O-连接糖肽,但很容易在整个蛋白质组样品中鉴定出来。使用高场非对称波形离子迁移谱(FAIMS)分离,我们显示出在高补偿电压(CVs)下,短脂族糖肽可以从复杂样品中富集,为鉴定基于亲水性富集的糖肽提供了另一种手段。结合整个蛋白质组和FAIMS分析,我们表明这些伯克霍尔德氏菌物种的可观察到的糖蛋白质组至少比最初认为的大25%。令人兴奋的是,使用FAIMS富集糖肽的能力似乎与弯曲杆菌胎儿亚种的N-连接糖肽普遍适用。胎儿也可以在高FAIMS CV条件下富集。综上所述,这些结果表明,FAIMS提供了一种访问糖肽的替代方法,并且是糖蛋白组学分析的宝贵工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号