当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dissolution of Minerals Driven by the Wetting Film in Rock Pores: Comparison of Silicates and Carbonates
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-11-01 , DOI: 10.1021/acsearthspacechem.0c00100 Tadashi Yokoyama 1 , Naoki Nishiyama 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-11-01 , DOI: 10.1021/acsearthspacechem.0c00100 Tadashi Yokoyama 1 , Naoki Nishiyama 2
Affiliation
|
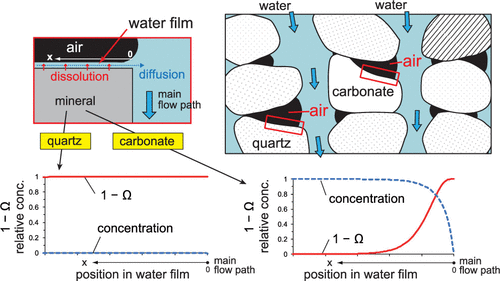
To evaluate how the presence of air in pores affects mineral dissolution, flow-through dissolution experiments were conducted using Berea sandstone, composed mainly of quartz, K-feldspar, Ca-Mg-Fe carbonates, and clays. Water was passed through a core both under the water-saturated condition and the dry-start condition, and the amounts of dissolved elements were compared. In the dry-start condition, after the onset of water flow, the volume fraction of air in pores initially became 38% and gradually decreased to 0% (fully saturated with water) over ∼9 days. As the air decreased, the bulk release rate of Ca in the dry-start condition approached those in the water-saturated condition, but the rates of the two conditions did not coincide until pores were fully filled with water. On the other hand, the bulk release rate of Si in the dry-start condition coincided with those in the water-saturated condition even though air was remaining in pores. Thus, the reduction of the amount of dissolution due to the presence of air was greater for carbonate than for silicates. Dissolution of a mineral exposed to the air in the pore is driven by a thin water film wetting the mineral surface. It is inferred that due to slow dissolution of quartz, dissolved Si was sufficiently flushed by diffusion in the water film and dissolution proceeded effectively even under the presence of air. In contrast, due to fast dissolution of carbonate, the flushing of Ca on the carbonate surface was insufficient and dissolution was significantly retarded due to the proximity to equilibrium in the film.
中文翻译:

湿膜驱动的矿物质在岩孔中的溶解:硅酸盐和碳酸盐的比较
为了评估孔隙中空气的存在如何影响矿物溶解,使用了主要由石英,钾长石,Ca-Mg-Fe碳酸盐和粘土组成的Berea砂岩进行了流通溶解实验。水在水饱和条件和干启动条件下都通过芯,并比较了溶解元素的量。在干启动条件下,水流开始后,孔中空气的体积分数最初变为38%,并在约9天之内逐渐降低至0%(完全充满水)。随着空气的减少,干启动条件下Ca的整体释放速率接近水饱和条件下的Ca的释放速率,但是在孔隙中完全充满水之前,这两个条件的速率并不吻合。另一方面,即使在孔中残留有空气,在干启动条件下Si的整体释放速率与在水饱和条件下的本体释放速率一致。因此,对于碳酸盐而言,由于存在空气而导致的溶解量的减少大于硅酸盐。暴露在空气中的矿物在孔隙中的溶解是由润湿矿物表面的薄水膜推动的。据推测,由于石英的缓慢溶解,溶解的Si通过在水膜中的扩散而被充分冲洗,并且即使在空气的存在下,溶解也有效地进行。相反,由于碳酸盐的快速溶解,Ca在碳酸盐表面上的冲洗不足,并且由于接近膜中的平衡而显着延迟了溶解。
更新日期:2020-11-19
中文翻译:

湿膜驱动的矿物质在岩孔中的溶解:硅酸盐和碳酸盐的比较
为了评估孔隙中空气的存在如何影响矿物溶解,使用了主要由石英,钾长石,Ca-Mg-Fe碳酸盐和粘土组成的Berea砂岩进行了流通溶解实验。水在水饱和条件和干启动条件下都通过芯,并比较了溶解元素的量。在干启动条件下,水流开始后,孔中空气的体积分数最初变为38%,并在约9天之内逐渐降低至0%(完全充满水)。随着空气的减少,干启动条件下Ca的整体释放速率接近水饱和条件下的Ca的释放速率,但是在孔隙中完全充满水之前,这两个条件的速率并不吻合。另一方面,即使在孔中残留有空气,在干启动条件下Si的整体释放速率与在水饱和条件下的本体释放速率一致。因此,对于碳酸盐而言,由于存在空气而导致的溶解量的减少大于硅酸盐。暴露在空气中的矿物在孔隙中的溶解是由润湿矿物表面的薄水膜推动的。据推测,由于石英的缓慢溶解,溶解的Si通过在水膜中的扩散而被充分冲洗,并且即使在空气的存在下,溶解也有效地进行。相反,由于碳酸盐的快速溶解,Ca在碳酸盐表面上的冲洗不足,并且由于接近膜中的平衡而显着延迟了溶解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号