当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anion‐regulated binding selectivity of Cr( III ) in collagen
Biopolymers ( IF 2.9 ) Pub Date : 2020-11-01 , DOI: 10.1002/bip.23406 Yi Zhang, Megha Mehta, Bradley W. Mansel, Hon Wei Ng, Yang Liu, Geoff Holmes, Eric C. Le Ru, Sujay Prabakar
Biopolymers ( IF 2.9 ) Pub Date : 2020-11-01 , DOI: 10.1002/bip.23406 Yi Zhang, Megha Mehta, Bradley W. Mansel, Hon Wei Ng, Yang Liu, Geoff Holmes, Eric C. Le Ru, Sujay Prabakar
|
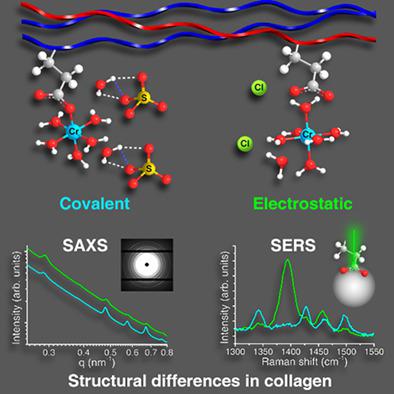
We present a mechanism for the selectivity of covalent/electrostatic binding of the Cr(III) ion to collagen, mediated by the kosmotropicity of the anions. Although a change in the long‐range ordered structure of collagen is observed after covalent binding (Cr(III)‐OOC) in the presence of SO42− at pH 4.5, the νsym(COO−) band remains intense, suggesting a relatively lower propensity for the Cr(III) to bind covalently instead of electrostatically through Cr(H2O)63+. Replacing SO42− with Cl− reduces the kosmotropic effect which further favors the electrostatic binding of Cr(III) to collagen. Our findings allow a greater understanding of mechanism‐specific metal binding in the collagen molecule. We also report for the first time, surface‐enhanced Raman spectroscopy to analyze binding mechanisms in collagen, suggesting a novel way to study chemical modifications in collagen‐based biomaterials.
中文翻译:

胶原中Cr(III)的阴离子调节结合选择性
我们提出了 Cr(III) 离子与胶原蛋白共价/静电结合的选择性机制,由阴离子的亲液性介导。尽管在 pH 4.5 的 SO42− 存在下共价结合 (Cr(III)-OOC) 后观察到胶原的长程有序结构发生变化,但 νsym(COO−) 带仍然强烈,表明相对较低的倾向Cr(III) 通过 Cr(H2O)63+ 共价结合而不是静电结合。用 Cl- 代替 SO42- 降低了亲液效应,这进一步有利于 Cr(III) 与胶原蛋白的静电结合。我们的发现使我们能够更好地了解胶原分子中机制特异性金属结合。我们还首次报告了表面增强拉曼光谱分析胶原蛋白的结合机制,
更新日期:2020-11-01
中文翻译:

胶原中Cr(III)的阴离子调节结合选择性
我们提出了 Cr(III) 离子与胶原蛋白共价/静电结合的选择性机制,由阴离子的亲液性介导。尽管在 pH 4.5 的 SO42− 存在下共价结合 (Cr(III)-OOC) 后观察到胶原的长程有序结构发生变化,但 νsym(COO−) 带仍然强烈,表明相对较低的倾向Cr(III) 通过 Cr(H2O)63+ 共价结合而不是静电结合。用 Cl- 代替 SO42- 降低了亲液效应,这进一步有利于 Cr(III) 与胶原蛋白的静电结合。我们的发现使我们能够更好地了解胶原分子中机制特异性金属结合。我们还首次报告了表面增强拉曼光谱分析胶原蛋白的结合机制,


























 京公网安备 11010802027423号
京公网安备 11010802027423号