当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure of the GDP‐bound GTPase domain of Rab5a from Leishmania donovani
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2053230x20013722 Muhammad Zohib 1 , Diva Maheshwari 1 , Ravi Kant Pal 2 , Stefanie Freitag-Pohl 3 , Bichitra Kumar Biswal 2 , Ehmke Pohl 3 , Ashish Arora 1
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2053230x20013722 Muhammad Zohib 1 , Diva Maheshwari 1 , Ravi Kant Pal 2 , Stefanie Freitag-Pohl 3 , Bichitra Kumar Biswal 2 , Ehmke Pohl 3 , Ashish Arora 1
Affiliation
|
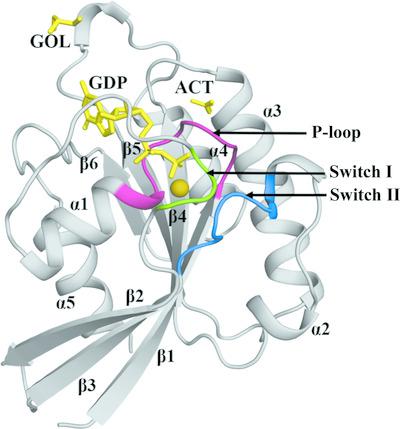
Eukaryotic Rab5s are highly conserved small GTPase‐family proteins that are involved in the regulation of early endocytosis. Leishmania donovani Rab5a regulates the sorting of early endosomes that are involved in the uptake of essential nutrients through fluid‐phase endocytosis. Here, the 1.80 Å resolution crystal structure of the N‐terminal GTPase domain of L. donovani Rab5a in complex with GDP is presented. The crystal structure determination was enabled by the design of specific single‐site mutations and two deletions that were made to stabilize the protein for previous NMR studies. The structure of LdRab5a shows the canonical GTPase fold, with a six‐stranded central mixed β‐sheet surrounded by five α‐helices. The positions of the Switch I and Switch II loops confirm an open conformation, as expected in the absence of the γ‐phosphate. However, in comparison to other GTP‐bound and GDP‐bound homologous proteins, the Switch I region traces a unique disposition in LdRab5a. One magnesium ion is bound to the protein at the GTP‐binding site. Molecular‐dynamics simulations indicate that the GDP‐bound structure exhibits higher stability than the apo structure. The GDP‐bound LdRab5a structure presented here will aid in efforts to unravel its interactions with its regulators, including the guanine nucleotide‐exchange factor, and will lay the foundation for a structure‐based search for specific inhibitors
中文翻译:

多诺瓦利什曼原虫 Rab5a 的 GDP 结合 GTPase 结构域的晶体结构
真核 Rab5s 是高度保守的小 GTP 酶家族蛋白,参与早期内吞作用的调节。Leishmania donovani Rab5a 调节早期内体的分类,这些内体参与通过液相内吞作用摄取必需营养素。这里,L. donovani的 N 端 GTPase 结构域的 1.80 Å 分辨率晶体结构提出了与 GDP 复合的 Rab5a。晶体结构的确定是通过设计特定的单位点突变和两个缺失来实现的,这些缺失是为了稳定蛋白质以用于之前的 NMR 研究。LdRab5a 的结构显示了典型的 GTPase 折叠,具有由五个 α 螺旋包围的六链中央混合 β-折叠。Switch I 和 Switch II 环的位置证实了开放构象,正如在没有 γ-磷酸盐的情况下所预期的那样。然而,与其他 GTP 结合和 GDP 结合的同源蛋白相比,Switch I 区域在 LdRab5a 中具有独特的倾向。一个镁离子在 GTP 结合位点与蛋白质结合。分子动力学模拟表明 GDP 结合结构比 apo 结构表现出更高的稳定性。
更新日期:2020-11-02
中文翻译:

多诺瓦利什曼原虫 Rab5a 的 GDP 结合 GTPase 结构域的晶体结构
真核 Rab5s 是高度保守的小 GTP 酶家族蛋白,参与早期内吞作用的调节。Leishmania donovani Rab5a 调节早期内体的分类,这些内体参与通过液相内吞作用摄取必需营养素。这里,L. donovani的 N 端 GTPase 结构域的 1.80 Å 分辨率晶体结构提出了与 GDP 复合的 Rab5a。晶体结构的确定是通过设计特定的单位点突变和两个缺失来实现的,这些缺失是为了稳定蛋白质以用于之前的 NMR 研究。LdRab5a 的结构显示了典型的 GTPase 折叠,具有由五个 α 螺旋包围的六链中央混合 β-折叠。Switch I 和 Switch II 环的位置证实了开放构象,正如在没有 γ-磷酸盐的情况下所预期的那样。然而,与其他 GTP 结合和 GDP 结合的同源蛋白相比,Switch I 区域在 LdRab5a 中具有独特的倾向。一个镁离子在 GTP 结合位点与蛋白质结合。分子动力学模拟表明 GDP 结合结构比 apo 结构表现出更高的稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号