当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural characterization of a nonhydrolyzing UDP‐GlcNAc 2‐epimerase from Neisseria meningitidis serogroup A
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2053230x20013680 Nicholas K Hurlburt 1 , Jasper Guan 1 , Hoonsan Ong 1 , Hai Yu 1 , Xi Chen 1 , Andrew J Fisher 1
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-11-02 , DOI: 10.1107/s2053230x20013680 Nicholas K Hurlburt 1 , Jasper Guan 1 , Hoonsan Ong 1 , Hai Yu 1 , Xi Chen 1 , Andrew J Fisher 1
Affiliation
|
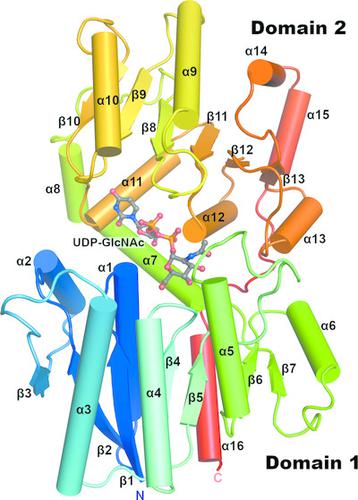
Bacterial nonhydrolyzing UDP‐N‐acetylglucosamine 2‐epimerases catalyze the reversible interconversion of UDP‐N‐acetylglucosamine (UDP‐GlcNAc) and UDP‐N‐acetylmannosamine (UDP‐ManNAc). UDP‐ManNAc is an important intermediate in the biosynthesis of certain cell‐surface polysaccharides, including those in some pathogenic bacteria, such as Neisseria meningitidis and Streptococcus pneumoniae. Many of these epimerases are allosterically regulated by UDP‐GlcNAc, which binds adjacent to the active site and is required to initiate UDP‐ManNAc epimerization. Here, two crystal structures of UDP‐N‐acetylglucosamine 2‐epimerase from Neisseria meningitidis serogroup A (NmSacA) are presented. One crystal structure is of the substrate‐free enzyme, while the other structure contains UDP‐GlcNAc substrate bound to the active site. Both structures form dimers as seen in similar epimerases, and substrate binding to the active site induces a large conformational change in which two Rossmann‐like domains clamp down on the substrate. Unlike other epimerases, NmSacA does not require UDP‐GlcNAc to instigate the epimerization of UDP‐ManNAc, although UDP‐GlcNAc was found to enhance the rate of epimerization. In spite of the conservation of residues involved in binding the allosteric UDP‐GlcNAc observed in similar UDP‐GlcNAc 2‐epimerases, the structures presented here do not contain UDP‐GlcNAc bound in the allosteric site. These structural results provide additional insight into the mechanism and regulation of this critical enzyme and improve the structural understanding of the ability of NmSacA to epimerize modified substrates.
中文翻译:

脑膜炎奈瑟菌 A 群非水解 UDP-GlcNAc 2-差向异构酶的结构表征
细菌非水解 UDP- N-乙酰氨基葡萄糖 2-差向异构酶催化 UDP- N-乙酰氨基葡萄糖 (UDP-GlcNAc) 和 UDP- N-乙酰甘露糖胺 (UDP-ManNAc) 的可逆相互转化。UDP-ManNAc 是某些细胞表面多糖生物合成的重要中间体,包括一些病原菌(如脑膜炎奈瑟菌和肺炎链球菌)中的多糖。许多这些差向异构酶受到 UDP-GlcNAc 的变构调节,UDP-GlcNAc 与活性位点相邻,并且是启动 UDP-ManNAc 差向异构化所必需的。在这里,提出了来自A 群脑膜炎奈瑟菌 (NmSacA) 的 UDP- N-乙酰氨基葡萄糖 2-差向异构酶(NmSacA ) 的两种晶体结构。一种晶体结构是无底物酶,而另一种结构包含与活性位点结合的 UDP-GlcNAc 底物。两种结构都形成二聚体,如在类似的差向异构酶中所见,底物与活性位点的结合会引起大的构象变化,其中两个罗斯曼样结构域夹紧底物。与其他差向异构酶不同,NmSacA 不需要 UDP-GlcNAc 来引发 UDP-ManNAc 的差向异构化,尽管发现 UDP-GlcNAc 可以提高差向异构化的速率。尽管在类似的 UDP-GlcNAc 2-差向异构酶中观察到与变构 UDP-GlcNAc 结合相关的残基是保守的,但此处呈现的结构不包含在变构位点中结合的 UDP-GlcNAc。这些结构结果提供了对这种关键酶的机制和调节的额外见解,并提高了对 NmSacA 差向异构化修饰底物能力的结构理解。
更新日期:2020-11-02
中文翻译:

脑膜炎奈瑟菌 A 群非水解 UDP-GlcNAc 2-差向异构酶的结构表征
细菌非水解 UDP- N-乙酰氨基葡萄糖 2-差向异构酶催化 UDP- N-乙酰氨基葡萄糖 (UDP-GlcNAc) 和 UDP- N-乙酰甘露糖胺 (UDP-ManNAc) 的可逆相互转化。UDP-ManNAc 是某些细胞表面多糖生物合成的重要中间体,包括一些病原菌(如脑膜炎奈瑟菌和肺炎链球菌)中的多糖。许多这些差向异构酶受到 UDP-GlcNAc 的变构调节,UDP-GlcNAc 与活性位点相邻,并且是启动 UDP-ManNAc 差向异构化所必需的。在这里,提出了来自A 群脑膜炎奈瑟菌 (NmSacA) 的 UDP- N-乙酰氨基葡萄糖 2-差向异构酶(NmSacA ) 的两种晶体结构。一种晶体结构是无底物酶,而另一种结构包含与活性位点结合的 UDP-GlcNAc 底物。两种结构都形成二聚体,如在类似的差向异构酶中所见,底物与活性位点的结合会引起大的构象变化,其中两个罗斯曼样结构域夹紧底物。与其他差向异构酶不同,NmSacA 不需要 UDP-GlcNAc 来引发 UDP-ManNAc 的差向异构化,尽管发现 UDP-GlcNAc 可以提高差向异构化的速率。尽管在类似的 UDP-GlcNAc 2-差向异构酶中观察到与变构 UDP-GlcNAc 结合相关的残基是保守的,但此处呈现的结构不包含在变构位点中结合的 UDP-GlcNAc。这些结构结果提供了对这种关键酶的机制和调节的额外见解,并提高了对 NmSacA 差向异构化修饰底物能力的结构理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号