Geoscience Frontiers ( IF 8.9 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.gsf.2020.10.001 Sudarsan Sahu , Utpal Gogoi , N.C. Nayak
|
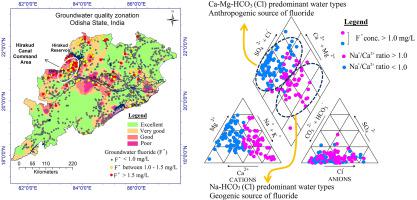
The work investigates the major solute chemistry of groundwater and fluoride enrichment (Fˉ) in the shallow phreatic aquifer of Odisha. The study also interprets the hydrogeochemical processes of solute acquisition and the genetic behavior of groundwater Fˉ contamination. A total of 1105 groundwater samples collected from across the state from different hydro-geomorphic settings have been analyzed for the major solutes and Fˉ content. Groundwater is alkaline in nature (range of pH: 6.6–8.7; ave.: 7.9) predominated by moderately hard to very hard types. Average cation and anion chemistry stand in the orders of Ca2+ > Na+ > Mg2+ > K+ and HCO3ˉ > Clˉ > SO42ˉ > CO32ˉ respectively. The average mineralization is low (319 mg/L). The primary water types are Ca-Mg-HCO3 and Ca-Mg-Cl-HCO3, followed by Na-Cl, Ca-Mg-Cl, and Na-Ca-Mg-HCO3-Cl. Silicate-halite dissolution and reverse ion exchange are the significant processes of solute acquisition.
Both the geogenic as well as the anthropogenic sources contribute to the groundwater fluoride contamination, etc. The ratio of Na+/Ca2+ > 1.0 comprises Na-HCO3 (Cl) water types with Fˉ > 1.0 mg/L (range 1.0–3.5 mg/L) where the Fˉ bears geogenic source. Positive relations exist between Fˉ and pH, Na+, TDS, and HCO3ˉ. It also reflects a perfect Na-TDS correlation (0.85). The ratio of Na+/Ca2+ < 1.0 segregates the sample population (Fˉ range: 1.0–4.0 mg/L) with the Fˉ derived from anthropogenic sources. Such water types include Ca-Mg-HCO3 (Cl) varieties which are recently recharged meteoritic water types. The Fˉ levels exhibit poor and negative correlations with the solutes in groundwater. The Na-TDS relation remains poor (0.12). In contrast, the TDS levels show strong correlations with Ca2+ (0.91), Mg2+ (0.80) and even Clˉ (0.91). The majority of the monitoring points with the anthropogenic sources of groundwater Fˉ are clustered in the Hirakud Canal Command area in the western parts of the state, indicating the role of irrigation return flow in the Fˉ contamination.
中文翻译:

印度奥里萨邦潜水含水层中的地下水溶质化学,水文地球化学过程和氟化物污染
这项工作研究了奥里萨邦浅层潜水含水层中地下水的主要溶质化学和氟化物富集(Fˉ)。该研究还解释了溶质获取的水文地球化学过程以及地下水Fˉ污染的遗传行为。分析了全州从不同水文地貌环境收集的总共1105个地下水样品的主要溶质和F 3含量。地下水本质上是碱性的(pH范围:6.6-8.7;平均:7.9),以中硬至极硬类型为主。平均阳离子和阴离子化学立场在Ca的订单2+ >的Na + >镁2+ >ķ +和HCO 3 ˉ> CL> SO 4 2 ˉ> CO3 2分别ˉ。平均矿化度低(319 mg / L)。主要水类型为Ca-Mg-HCO 3和Ca-Mg-Cl-HCO 3,其次为Na-Cl,Ca-Mg-Cl和Na-Ca-Mg-HCO 3 -Cl。硅酸盐-卤酸盐的溶解和反向离子交换是溶质获取的重要过程。
地源和人为来源均对地下水中的氟化物造成污染等。Na + / Ca 2+ > 1.0的比率包括Fˉ> 1.0 mg / L的Na-HCO 3(Cl)水类型(范围1.0– 3.5 mg / L),其中Fˉ带有地源。F +与pH,Na +,TDS和HCO 3-之间存在正相关关系。它也反映了完美的Na-TDS相关性(0.85)。Na + / Ca 2+ <1.0的比率将样本人口与Fˉ分离为人为来源(Fˉ范围:1.0–4.0 mg / L)。这些水类型包括Ca-Mg-HCO 3(Cl)品种,这些品种最近充斥了流水质的水。Fˉ水平与地下水中的溶质表现出不良的负相关性。Na-TDS关系仍然很差(0.12)。相反,TDS水平与Ca 2+(0.91),Mg 2+(0.80)甚至Clˉ(0.91)有很强的相关性。大部分人为地下水Fˉ的监测点都聚集在该州西部的Hirakud运河指挥区,表明灌溉回流在Fˉ污染中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号