Developmental Biology ( IF 2.7 ) Pub Date : 2020-11-02 , DOI: 10.1016/j.ydbio.2020.10.012 Rahyza I.F. Assis , Geórgia da S. Feltran , Maria Eduarda Salomão Silva , Iasmin Caroline do Rosário Palma , Emanuel Silva Rovai , Taís Browne de Miranda , Marcel Rodrigues Ferreira , Willian F. Zambuzzi , Alexander Birbrair , Denise C. Andia , Rodrigo A. da Silva
|
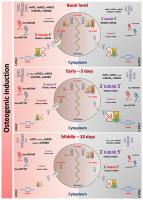
Mesenchymal stem cells are candidates for therapeutic strategies in periodontal repair due to their osteogenic potential. In this study, we identified epigenetic markers during osteogenic differentiation, taking advantage of the individual pattern of mesenchymal cells of the periodontal ligament with high (h-PDLCs) and low (l-PDLCs) osteogenic capacity. We found that the involvement of non-coding RNAs in the regulation of the RUNX2 gene is strongly associated with high osteogenic potential. Moreover, we evaluated miRs and genes that encode enzymes to process miRs and their biogenesis. Our data show the high expression of the XPO5 gene, and miRs 7 and 22 observed in the l-PDLCs might be involved in acquiring osteogenic potential, suppressing RUNX2 gene expression. Further, an inversely proportional correlation between lncRNAs (HOTAIR and HOTTIP) and RUNX2 gene expression was observed in both l- and h-PDLCs, and it was also related to the distinct osteogenic phenotypes. Thus, our results indicate the low expression of XPO5 in h-PDLC might be the limiting point for blocking the miRs biogenesis, allowing the high gene expression of RUNX2. In accordance, the low expression of miRs, HOTAIR, and HOTTIP could be a prerequisite for increased osteogenic potential in h-PDLCs. These results will help us to better understand the underlying mechanisms of osteogenesis, considering the heterogeneity in the osteogenic potential of PDLCs that might be related to a distinct transcriptional profile of lncRNAs and the biogenesis machinery.
中文翻译:

牙周膜间充质干细胞成骨表型获得过程中RUNX2转录后加工中非编码RNA的阻遏作用。
间充质干细胞由于具有成骨作用,因此是牙周修复治疗策略的候选者。在这项研究中,我们利用高(h-PDLCs)和低(l-PDLCs)成骨能力的牙周膜间充质细胞的个体模式,在成骨分化过程中鉴定了表观遗传标记。我们发现,非编码RNA参与RUNX2基因的调节与高成骨潜能密切相关。此外,我们评估了miRs和编码酶以处理miRs及其生物发生的基因。我们的数据显示XPO5的高表达I-PDLCs中观察到的miRs 7和22可能参与获得成骨潜能,从而抑制RUNX2基因表达。此外,在l -PDLC和h-PDLC中均观察到lncRNA(HOTAIR和HOTTIP)与RUNX2基因表达之间成反比的相关性,并且还与不同的成骨表型有关。因此,我们的结果表明,h-PDLC中XPO5的低表达可能是阻断miRs生物发生的限制点,从而允许RUNX的高基因表达2.因此,miRs,HOTAIR和HOTTIP的低表达可能是h-PDLC中成骨潜能增加的先决条件。这些结果将帮助我们更好地了解成骨的潜在机制,考虑到PDLC的成骨潜力的异质性可能与lncRNA的独特转录谱和生物发生机制有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号