Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2020-11-02 , DOI: 10.1016/j.cej.2020.127550 Jongsik Kim , Yun Jeong Choe , Sang Hoon Kim
|
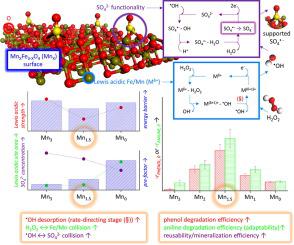
Generation of SO4•- anchored on metal oxides via radical transfer from •OH to surface SO42- functionality (•OH → SO4•-) is singular, unraveled recently, and promising to decompose aqueous refractory contaminants. The core in furthering supported SO4•- production is to reduce the energy required to accelerate the rate-determining step of the •OH → SO4•- (•OH desorption), while increasing the collision frequency between the •OH precursors (H2O2) and H2O2 activators (Lewis acidic metals) or between SO42--attacking radicals (•OH) and supported SO4•- precursors (SO42-). Herein, Mn-substituted Fe3O4 oxo-spinels (MnXFe3-XO4; MnX) served as reservoirs to accommodate the Lewis acidic Fe/Mn and SO42-, whose properties were tailored by altering the metal compositions (X). The production of supported SO4•- via the •OH → SO4•- was of high tangibility, as confirmed by their electron paramagnetic resonance spectra coupled with those simulated. A concave trend was observed in the plot of the Lewis acidic strength of Fe/Mn versus X of MnX with the minimum at X∼ 1.5. Hence, Mn1.5 could expedite •OH liberation from the surface most proficiently and therefore exhibited the greatest initial H2O2 scission rate, as corroborated by its lowest energy barrier needed for activating the •OH → SO4•-. Meanwhile, a volcano-shaped trend was found in the plot of SO42- concentration versus X of MnX (other than Mn3). This could tentatively increase the collision frequency between •OH and SO42- on the surface of Mn1.5, as partially substantiated by its second largest pre-factor among the catalysts. Therefore, Mn1.5 exhibited the highest phenol consumption rate (-rPHENOL, 0) among the catalysts, which was ∼20-fold larger than those for SO42--modified Fe2O3 and NiO, which we reported previously. Mn1.5 also outperformed other catalysts in recycling phenol degradation, fragmenting another pollutant (aniline), and mineralizing phenol/aniline.
中文翻译:

量身定制刘易斯酸性金属和SO 4 2-在双金属Mn-Fe氧-尖晶石上的功能,以利用支持的SO 4 •在水污染物裂解中的应用
的SO代4 • -通过从自由基转移锚固在金属氧化物• OH表面SO 4 2-的功能(• OH→SO 4 • - )是单数,最近解开,并有希望的分解含水耐火污染物。进一步促进支持的SO 4 •-生产的核心是减少加速• OH→SO 4 •-(- • OH解吸)的速率确定步骤所需的能量,同时增加• OH前体(H 2 O 2)和H 2O 2活化剂(路易斯酸性金属)或在SO 4 2-攻击自由基(• OH)与负载的SO 4 •-前体(SO 4 2-)之间。此处,Mn取代的Fe 3 O 4氧代尖晶石(Mn X Fe 3-X O 4; Mn X)用作储存路易斯酸性Fe / Mn和SO 4 2-的储层,其性质是通过改变金属来调整的成分(X)。生产支持SO 4 • -经由• OH→SO 4 • -由它们的电子顺磁共振光谱和模拟的光谱证实,其具有较高的可塑性。Fe / Mn的路易斯酸性强度对Mn X的X的曲线中观察到凹形趋势,其最小值在X〜1.5。因此,Mn 1.5可以最充分地促进• OH从表面的释放,因此表现出最大的初始H 2 O 2断裂速率,这由活化• OH→SO 4 •-所需的最低能垒所证实。同时,在SO 4 2-浓度与Mn X(Mn以外的X)的曲线图中发现了火山状的趋势。3)。这可能会暂时增加Mn 1.5表面上• OH与SO 4 2-之间的碰撞频率,这在某种程度上被其在催化剂中的第二大前置因子所证实。因此,Mn 1.5表现出最高的苯酚消耗率(-r PHENOL,0),比我们先前报道的SO 4 2-改性的Fe 2 O 3和NiO的约高20倍。锰1.5在循环苯酚降解,破碎另一种污染物(苯胺)和使苯酚/苯胺矿化方面也胜过其他催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号