Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570180817999200630125449 Himesh Makala 1 , Venkatasubramanian Ulaganathan 1 , Aravind Sivasubramanian 1 , Narendran Rajendran 1 , Shankar Subramanian 1
|
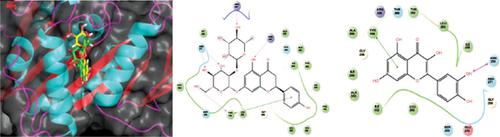
Background: Human mitotic kinesins play an essential role in mitotic cell division. Targeting the spindle separation phase of mitosis has gained much attention in cancer chemotherapy. Spindle segregation is carried out mainly by the kinesin, Eg5. Many Eg5 inhibitors are in different phases of clinical trials as cancer drugs. This enzyme has two allosteric binding sites to which the inhibitors can bind. The first site is formed by loop L5, helix α2 and helix α3 and all the current drug candidates bind un-competitively to this site with ATP/ADP. The second site, formed by helix α4 and helix α6, which has gained attention recently, has not been explored well. Some inhibitors that bind to this site are competitive, while others are uncompetitive to ATP/ADP. Phenylpropanoids are pharmacologically active secondary metabolites.
Methods: In this study, we have evaluated fourteen phenyl propanoids extracted from Citrus medica for inhibitory activity against human mitotic kinesin Eg5 in vitro steady-state ATPase assay. Ther interactions and stability using molecular docking and molecular dynamics simulations.
Results and Discussions: Of the fourteen compounds tested, naringin and quercetin showed good activity with IC50 values in the micromolar range. Molecular docking studies of these complexes showed that both the molecules interact with the key residues of the active site predominantly thorough hydrophobic & aromatic π–π interactions consistent with the known inhibitors. Besides, these molecules also form hydrogen bonding interactions stabilizing the complexes. Molecular dynamics simulations of these complexes confirm the stability of these interactions.
Conclusion: These results can be used as a strong basis for further modification of these compounds to design new inhibitors with higher potency using structure-based drug design.
中文翻译:

评价从柑桔中分离的苯基丙烷作为有丝分裂驱动蛋白Eg5的潜在抑制剂。
背景:人类有丝分裂驱动蛋白在有丝分裂细胞分裂中起着至关重要的作用。靶向有丝分裂的纺锤体分离期已在癌症化学疗法中引起了广泛关注。主轴分离主要由驱动蛋白Eg5进行。许多Eg5抑制剂作为抗癌药物处于临床试验的不同阶段。该酶具有抑制剂可以结合的两个变构结合位点。第一个位点由环L5,螺旋α2和螺旋α3形成,所有当前候选药物都通过ATP / ADP非竞争性地与该位结合。由螺旋α4和螺旋α6形成的第二个位点最近没有引起人们的注意,但尚未得到很好的探索。某些与该位点结合的抑制剂具有竞争性,而另一些则与ATP / ADP不竞争。苯丙烷是具有药理活性的次级代谢产物。
方法:在这项研究中,我们评估了从柑橘中提取的十四种苯基丙烷类化合物在体外稳态ATPase检测中对人有丝分裂驱动蛋白Eg5的抑制活性。使用分子对接和分子动力学模拟可以实现相互作用和稳定性。
结果与讨论:在测试的14种化合物中,柚皮苷和槲皮素表现出良好的活性,IC50值在微摩尔范围内。这些复合物的分子对接研究表明,两个分子均与活性位点的关键残基相互作用,主要是与已知抑制剂形成的彻底的疏水性和芳香族π-π相互作用。此外,这些分子还形成氢键相互作用以稳定配合物。这些配合物的分子动力学模拟证实了这些相互作用的稳定性。
结论:这些结果可作为进一步修饰这些化合物以使用基于结构的药物设计设计具有更高效力的新抑制剂的有力依据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号