当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hopping in High Concentration Electrolytes - Long Time Bulk and Single-Particle Signatures, Free Energy Barriers, and Structural Insights
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-10-30 , DOI: 10.1021/acs.jpclett.0c02995 Srimayee Mukherji 1 , Nikhil V. S. Avula 1 , Rahul Kumar 1 , Sundaram Balasubramanian 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-10-30 , DOI: 10.1021/acs.jpclett.0c02995 Srimayee Mukherji 1 , Nikhil V. S. Avula 1 , Rahul Kumar 1 , Sundaram Balasubramanian 1
Affiliation
|
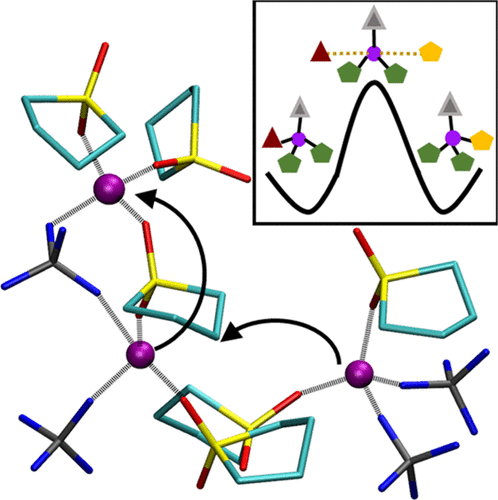
Although ion-hopping is believed to be a significant mode of transport for small ions in liquid high concentration electrolytes (HCE), its bulk signatures over sufficiently long time intervals are yet to be shown. We computationally establish the long and short time imprints of hopping in HCEs using LiBF4-in-sulfolane mixtures as models. The high viscosity of this electrolyte leads to significant dynamic heterogeneity in Li-ion transport. Li-ions exhibit a preference to transit to previously occupied Li-ion-sites, bridged through anion or solvent molecules. Hopping in the liquid matrix was found to be an activated process, whose free energy barrier and transition state structure have been determined. Evidence for nanoscale compositional heterogeneity at high salt concentrations is also presented. The simulations shed light on the composition, stiffness, and lifetime of the solvation shell of Li ions. The understanding of HCEs gleaned from this study will spearhead the choice, engineering and applicability of this class of electrolytes.
中文翻译:

高浓度电解质的跳跃-长时间散装和单颗粒签名,自由能垒和结构见解
尽管离子跳跃被认为是液态高浓度电解质(HCE)中小离子的一种重要传输方式,但在足够长的时间间隔内其体相特征仍有待显示。我们使用LiBF 4通过计算建立了HCE中跳跃的长时间和短时印记-环丁砜混合物中的模型。这种电解质的高粘度导致锂离子运输中明显的动态异质性。锂离子表现出优先过渡到通过阴离子或溶剂分子桥接的先前占据的锂离子位点的能力。发现在液体基质中跳跃是一个激活的过程,其自由能垒和过渡态结构已经确定。还提供了在高盐浓度下纳米级成分异质性的证据。这些模拟揭示了锂离子溶剂化壳的组成,硬度和寿命。从这项研究中收集到的对HCE的理解将引领此类电解质的选择,工程设计和适用性。
更新日期:2020-11-19
中文翻译:

高浓度电解质的跳跃-长时间散装和单颗粒签名,自由能垒和结构见解
尽管离子跳跃被认为是液态高浓度电解质(HCE)中小离子的一种重要传输方式,但在足够长的时间间隔内其体相特征仍有待显示。我们使用LiBF 4通过计算建立了HCE中跳跃的长时间和短时印记-环丁砜混合物中的模型。这种电解质的高粘度导致锂离子运输中明显的动态异质性。锂离子表现出优先过渡到通过阴离子或溶剂分子桥接的先前占据的锂离子位点的能力。发现在液体基质中跳跃是一个激活的过程,其自由能垒和过渡态结构已经确定。还提供了在高盐浓度下纳米级成分异质性的证据。这些模拟揭示了锂离子溶剂化壳的组成,硬度和寿命。从这项研究中收集到的对HCE的理解将引领此类电解质的选择,工程设计和适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号