Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Roadmap to the Bioanalytical Testing of COVID-19: From Sample Collection to Disease Surveillance
ACS Sensors ( IF 8.9 ) Pub Date : 2020-10-30 , DOI: 10.1021/acssensors.0c01377 Amin Hosseini 1 , Richa Pandey 2 , Enas Osman 1 , Amanda Victorious 1 , Feng Li 3, 4 , Tohid Didar 1, 5 , Leyla Soleymani 1, 2
ACS Sensors ( IF 8.9 ) Pub Date : 2020-10-30 , DOI: 10.1021/acssensors.0c01377 Amin Hosseini 1 , Richa Pandey 2 , Enas Osman 1 , Amanda Victorious 1 , Feng Li 3, 4 , Tohid Didar 1, 5 , Leyla Soleymani 1, 2
Affiliation
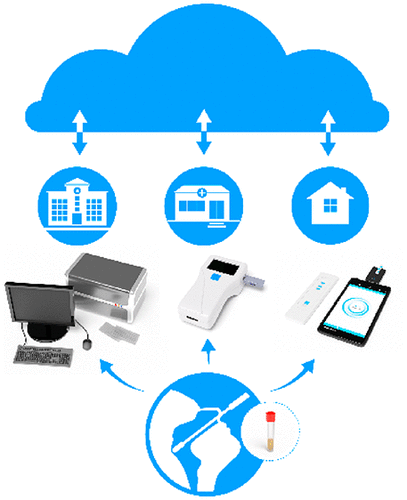
|
The disease caused by SARS-CoV-2, coronavirus disease 2019 (COVID-19), has led to a global pandemic with tremendous mortality, morbidity, and economic loss. The current lack of effective vaccines and treatments places tremendous value on widespread screening, early detection, and contact tracing of COVID-19 for controlling its spread and minimizing the resultant health and societal impact. Bioanalytical diagnostic technologies have played a critical role in the mitigation of the COVID-19 pandemic and will continue to be foundational in the prevention of the subsequent waves of this pandemic along with future infectious disease outbreaks. In this Review, we aim at presenting a roadmap to the bioanalytical testing of COVID-19, with a focus on the performance metrics as well as the limitations of various techniques. The state-of-the-art technologies, mostly limited to centralized laboratories, set the clinical metrics against which the emerging technologies are measured. Technologies for point-of-care and do-it-yourself testing are rapidly emerging, which open the route for testing in the community, at home, and at points-of-entry to widely screen and monitor individuals for enabling normal life despite of an infectious disease pandemic. The combination of different classes of diagnostic technologies (centralized and point-of-care and relying on multiple biomarkers) are needed for effective diagnosis, treatment selection, prognosis, patient monitoring, and epidemiological surveillance in the event of major pandemics such as COVID-19.
中文翻译:

COVID-19 生物分析检测路线图:从样本采集到疾病监测
由 SARS-CoV-2 引起的疾病,即 2019 年冠状病毒病 (COVID-19),已导致全球大流行,造成巨大的死亡率、发病率和经济损失。目前缺乏有效的疫苗和治疗方法,因此对 COVID-19 进行广泛筛查、早期检测和接触者追踪对于控制其传播并最大程度地减少由此产生的健康和社会影响具有巨大价值。生物分析诊断技术在缓解 COVID-19 大流行方面发挥了关键作用,并将继续成为预防这一大流行的后续浪潮以及未来传染病爆发的基础。在这篇综述中,我们的目标是提出 COVID-19 生物分析测试的路线图,重点关注性能指标以及各种技术的局限性。最先进的技术大多局限于集中实验室,它们设定了衡量新兴技术的临床指标。现场护理和自助检测技术正在迅速兴起,这为在社区、家庭和入境点进行检测开辟了途径,以广泛筛查和监测个人,以实现正常生活,尽管传染病大流行。在发生 COVID-19 等重大流行病时,需要结合不同类别的诊断技术(集中式和现场护理并依赖多种生物标志物)来进行有效的诊断、治疗选择、预后、患者监测和流行病学监测。
更新日期:2020-11-25
中文翻译:

COVID-19 生物分析检测路线图:从样本采集到疾病监测
由 SARS-CoV-2 引起的疾病,即 2019 年冠状病毒病 (COVID-19),已导致全球大流行,造成巨大的死亡率、发病率和经济损失。目前缺乏有效的疫苗和治疗方法,因此对 COVID-19 进行广泛筛查、早期检测和接触者追踪对于控制其传播并最大程度地减少由此产生的健康和社会影响具有巨大价值。生物分析诊断技术在缓解 COVID-19 大流行方面发挥了关键作用,并将继续成为预防这一大流行的后续浪潮以及未来传染病爆发的基础。在这篇综述中,我们的目标是提出 COVID-19 生物分析测试的路线图,重点关注性能指标以及各种技术的局限性。最先进的技术大多局限于集中实验室,它们设定了衡量新兴技术的临床指标。现场护理和自助检测技术正在迅速兴起,这为在社区、家庭和入境点进行检测开辟了途径,以广泛筛查和监测个人,以实现正常生活,尽管传染病大流行。在发生 COVID-19 等重大流行病时,需要结合不同类别的诊断技术(集中式和现场护理并依赖多种生物标志物)来进行有效的诊断、治疗选择、预后、患者监测和流行病学监测。


























 京公网安备 11010802027423号
京公网安备 11010802027423号