当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalytic α-Alkylation of Amines with Alkyl Halides
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-10-30 , DOI: 10.1021/acscatal.0c04519 Lingying Leng 1 , Joseph M. Ready 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-10-30 , DOI: 10.1021/acscatal.0c04519 Lingying Leng 1 , Joseph M. Ready 1
Affiliation
|
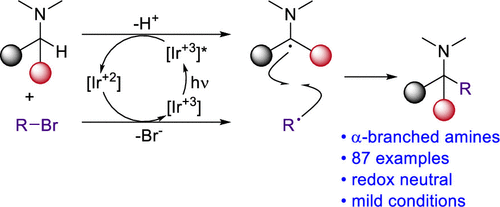
α-Branched amines represent essential building blocks for organic synthesis. They are traditionally prepared through nucleophilic addition to imines. These methods often require highly reactive organometallic reagents and proceed under rigorous air- and moisture-free conditions. Here we describe an alternative approach that involves a C-alkylation of amines with alkyl bromides. Mechanistically, the reaction likely involves photocatalytic generation of an α-amino radical and a stabilized carbon-centered radical (allyl, benzyl, α-carbonyl) followed by radical recombination. This approach offers a mild, atom-economical, redox neutral synthesis of α-branched amines that shows broad scope and avoids premetalated reagents.
中文翻译:

胺与烷基卤化物的光催化α-烷基化
α-支化胺代表了有机合成的基本组成部分。传统上,它们是通过亲核加成亚胺制备的。这些方法通常需要高反应性的有机金属试剂,并在严格的无空气和湿气条件下进行。在这里,我们描述了一种替代方法,该方法涉及将胺与烷基溴的C-烷基化。从机理上讲,该反应可能涉及光催化生成α-氨基自由基和稳定的以碳为中心的自由基(烯丙基,苄基,α-羰基),然后进行自由基重组。这种方法提供了温和的,原子经济的,氧化还原中性的α-支化胺合成方法,该方法显示范围广,并且避免了预金属化试剂。
更新日期:2020-11-21
中文翻译:

胺与烷基卤化物的光催化α-烷基化
α-支化胺代表了有机合成的基本组成部分。传统上,它们是通过亲核加成亚胺制备的。这些方法通常需要高反应性的有机金属试剂,并在严格的无空气和湿气条件下进行。在这里,我们描述了一种替代方法,该方法涉及将胺与烷基溴的C-烷基化。从机理上讲,该反应可能涉及光催化生成α-氨基自由基和稳定的以碳为中心的自由基(烯丙基,苄基,α-羰基),然后进行自由基重组。这种方法提供了温和的,原子经济的,氧化还原中性的α-支化胺合成方法,该方法显示范围广,并且避免了预金属化试剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号