当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Photoelectrochemical Activity of α-Fe2O3−CdFe2O4 Hybrid Structure for the Water Oxidation Reaction
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2020-10-30 , DOI: 10.1002/ijch.202000059 Michael Volokh 1 , Mahmud Diab 2
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2020-10-30 , DOI: 10.1002/ijch.202000059 Michael Volokh 1 , Mahmud Diab 2
Affiliation
|
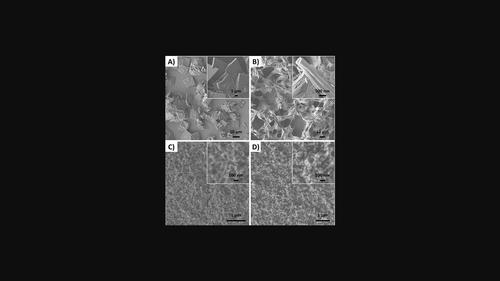
The electrochemical properties of spinel ferrites have been studied since the 1970s after the pioneering discovery of water electrolysis by using TiO2 as the photoanode in a photoelectrochemical cell. Several approaches have been developed to fabricate ferrites of different sizes and shapes but most of these methods suffer from several disadvantages, such as the difficulty to produce the ferrite in a thin layer form or on top of another (pre-prepared) structure as a second layer. Moreover, the formation process requires calcination at high temperatures, and the electrode fabrication requires an additional assembly method. Herein, a CdFe2O4 ferrite was formed via chemical vapor deposition of a single-source precursor directly on a hematite structure followed by an annealing process. This thermal treatment simultaneously achieves both activation of the hematite and the formation of CdFe2O4. Three different iron oxide (rectangular, nanobelt, and mesoporous) structures were examined as photoanode electrodes. The maximum current at 1.65 V vs. RHE of the mesoporous film was more than two times higher than the rectangular and the nanobelt films. Therefore, iron oxide with mesoporous structures was used to form the hybrid structure of α-Fe2O3−CdFe2O4. The hybrid structure presents stable and high photocurrent density (about 20 % enhancement in the measured maximum current density at 1.65 V vs. RHE).
中文翻译:

α-Fe2O3−CdFe2O4 杂化结构的合成及其用于水氧化反应的光电化学活性
自 20 世纪 70 年代开创性地发现使用 TiO 2作为光电化学电池中的光阳极进行水电解后,尖晶石铁氧体的电化学性能就得到了研究。已经开发了几种方法来制造不同尺寸和形状的铁氧体,但大多数这些方法都存在一些缺点,例如难以以薄层形式或在另一个(预先准备的)结构之上生产铁氧体作为第二个层。此外,形成过程需要高温煅烧,并且电极制造需要额外的组装方法。在此,通过直接在赤铁矿结构上化学气相沉积单源前体并随后进行退火工艺来形成CdFe 2 O 4铁氧体。该热处理同时实现了赤铁矿的活化和CdFe 2 O 4的形成。研究了三种不同的氧化铁(矩形、纳米带和介孔)结构作为光阳极电极。介孔膜在 1.65 V vs. RHE 下的最大电流比矩形膜和纳米带膜高两倍多。因此,采用具有介孔结构的氧化铁来形成α-Fe 2 O 3 -CdFe 2 O 4的杂化结构。该混合结构呈现出稳定且高的光电流密度(与 RHE 相比,在 1.65 V 下测得的最大电流密度提高了约 20%)。
更新日期:2020-10-30
中文翻译:

α-Fe2O3−CdFe2O4 杂化结构的合成及其用于水氧化反应的光电化学活性
自 20 世纪 70 年代开创性地发现使用 TiO 2作为光电化学电池中的光阳极进行水电解后,尖晶石铁氧体的电化学性能就得到了研究。已经开发了几种方法来制造不同尺寸和形状的铁氧体,但大多数这些方法都存在一些缺点,例如难以以薄层形式或在另一个(预先准备的)结构之上生产铁氧体作为第二个层。此外,形成过程需要高温煅烧,并且电极制造需要额外的组装方法。在此,通过直接在赤铁矿结构上化学气相沉积单源前体并随后进行退火工艺来形成CdFe 2 O 4铁氧体。该热处理同时实现了赤铁矿的活化和CdFe 2 O 4的形成。研究了三种不同的氧化铁(矩形、纳米带和介孔)结构作为光阳极电极。介孔膜在 1.65 V vs. RHE 下的最大电流比矩形膜和纳米带膜高两倍多。因此,采用具有介孔结构的氧化铁来形成α-Fe 2 O 3 -CdFe 2 O 4的杂化结构。该混合结构呈现出稳定且高的光电流密度(与 RHE 相比,在 1.65 V 下测得的最大电流密度提高了约 20%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号