当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium(III)‐Catalyzed Annulation of 2‐Arylimidazo[1,2‐a]pyridines with Maleimides: Synthesis of 1H‐Benzo[e]pyrido[1′,2′:1,2]imidazo[4,5‐g]isoindole‐1,3(2H)‐Diones and their Photophysical Studies
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-10-26 , DOI: 10.1002/adsc.202000960 Vikki N. Shinde 1 , Tapta Kanchan Roy 2 , Sonam Jaspal 1 , Dhananjay S. Nipate 1 , Neha Meena 1 , Krishnan Rangan 3 , Dalip Kumar 1 , Anil Kumar 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-10-26 , DOI: 10.1002/adsc.202000960 Vikki N. Shinde 1 , Tapta Kanchan Roy 2 , Sonam Jaspal 1 , Dhananjay S. Nipate 1 , Neha Meena 1 , Krishnan Rangan 3 , Dalip Kumar 1 , Anil Kumar 1
Affiliation
|
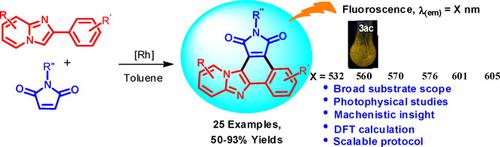
Rhodium(III)‐catalyzed dehydrogenative annulation of 2‐aryl‐imidazo[1,2‐a]pyridines with maleimides is described. The reaction afforded 1H‐benzo[e]pyrido[1′,2′:1,2]imidazo[4,5‐g]isoindole‐1,3(2H)‐diones in high yields with wide range of functional group tolerance. The reaction proceeds through Rh(III)‐catalyzed C−H bond activation, followed by maleimide insertion and intramolecular cyclization. Photophysical properties of 1H‐benzo[e]pyrido[1′,2′:1,2]imidazo[4,5‐g]isoindole‐1,3(2H)‐diones were studied with UV‐visible and fluorescence spectroscopy and validated by quantum chemical calculations. All the annulated products showed large Stokes shift values with emission in the range of 530–618 nm, and moderate to high quantum yields.
中文翻译:

铑(III)催化2-马来酰亚胺咪唑并[1,2-a]吡啶与马来酰亚胺的环合反应:1H-苯并[e]吡啶并[1',2':1,2,]咪唑[4,5-g]的合成isoindole‐1,3(2H)‐Diones及其光物理研究
描述了铑(III)催化的2-芳基咪唑并[1,2- a ]吡啶与马来酰亚胺的脱氢环化反应。反应以高收率提供了1 H-苯并[ e ]吡啶基[1',2':1,2]咪唑并[4,5 - g ]异吲哚-1,3(2H)-二酮,具有宽泛的官能团耐受性。反应通过Rh(III)催化的CH键活化,然后进行马来酰亚胺插入和分子内环化。1 H-苯并[ e ]吡啶并[1',2':1,2]咪唑[4,5- g ]的光物理性质用紫外可见光谱和荧光光谱研究了] isoindole-1,3(2H)-二酮,并通过量子化学计算进行了验证。所有的环状产物均显示出较大的斯托克斯位移值,且发射范围为530–618 nm,并且具有中等至高的量子产率。
更新日期:2020-12-22
中文翻译:

铑(III)催化2-马来酰亚胺咪唑并[1,2-a]吡啶与马来酰亚胺的环合反应:1H-苯并[e]吡啶并[1',2':1,2,]咪唑[4,5-g]的合成isoindole‐1,3(2H)‐Diones及其光物理研究
描述了铑(III)催化的2-芳基咪唑并[1,2- a ]吡啶与马来酰亚胺的脱氢环化反应。反应以高收率提供了1 H-苯并[ e ]吡啶基[1',2':1,2]咪唑并[4,5 - g ]异吲哚-1,3(2H)-二酮,具有宽泛的官能团耐受性。反应通过Rh(III)催化的CH键活化,然后进行马来酰亚胺插入和分子内环化。1 H-苯并[ e ]吡啶并[1',2':1,2]咪唑[4,5- g ]的光物理性质用紫外可见光谱和荧光光谱研究了] isoindole-1,3(2H)-二酮,并通过量子化学计算进行了验证。所有的环状产物均显示出较大的斯托克斯位移值,且发射范围为530–618 nm,并且具有中等至高的量子产率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号