Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-24 , DOI: 10.1016/j.tet.2020.131707 Michael A. Leitch , Gregory W. O’Neil
|
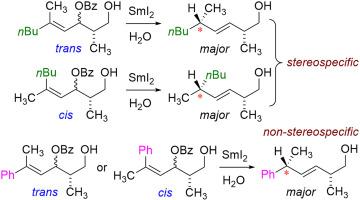
A series of experiments were performed to determine if SmI2(H2O)n reductions of allylic benzoates was stereospecific to alkene geometry. The results indicate that for alkyl-substituted alkenes, the reaction is stereospecific (i.e. the cis- and trans-isomers select for the opposite major diastereomer). However, the introduction of a phenyl substituent causes the reaction to become non-stereospecific, both cis- and trans-isomers converging to the same major diastereomer. A potential rationale is presented based on stabilization of a proposed organosamarium intermediate by the phenyl group that allows for isomerization of alkene geometry to a sterically preferred trans-configuration at the transition state. The data provide further insights into the mechanism of this reaction, the nature of allylic organosamarium complexes, and the unique reactivity of benzene rings in samarium-mediated transformations.
中文翻译:

sa介导的烯丙基苯甲酸酯还原反应中依赖于底物的立体特异性
进行了一系列实验,以确定烯丙基苯甲酸酯的SmI 2(H 2 O)n还原对烯烃几何形状是否具有立体特异性。结果表明,对于烷基取代的烯烃,该反应是立体特异性的(即顺式和反式异构体选择相反的主要非对映异构体)。但是,引入苯基取代基会使反应变成非立体特异性的,无论是顺式还是反式异构体收敛到相同的主要非对映异构体。基于通过苯基对提出的有机sa中间体的稳定化,提出了一种潜在的原理,其允许烯烃几何形状异构化为过渡态的空间优选的反式构型。数据为该反应的机理,烯丙基有机sa配合物的性质以及sa介导的转化中苯环的独特反应性提供了进一步的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号