Structure ( IF 5.7 ) Pub Date : 2020-10-22 , DOI: 10.1016/j.str.2020.10.002 Marie B Bertelsen 1 , Meriem Senissar 1 , Maja H Nielsen 1 , Francesco Bisiak 1 , Marta V Cunha 1 , Ashley L Molinaro 2 , Dayle A Daines 2 , Ditlev E Brodersen 1
|
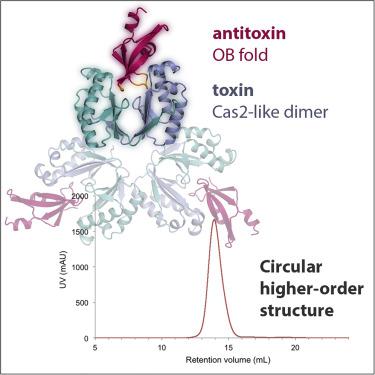
Bacterial type II toxin-antitoxin (TA) modules encode a toxic protein that downregulates metabolism and a specific antitoxin that binds and inhibits the toxin during normal growth. In non-typeable Haemophilus influenzae, a common cause of infections in humans, the vapXD locus was found to constitute a functional TA module and contribute to pathogenicity; however, the mode of action of VapD and the mechanism of inhibition by the VapX antitoxin remain unknown. Here, we report the structure of the intact H. influenzae VapXD complex, revealing an unusual 2:1 TA molecular stoichiometry where a Cas2-like homodimer of VapD binds a single VapX antitoxin. VapX consists of an oligonucleotide/oligosaccharide-binding domain that docks into an asymmetrical cavity on the toxin dimer. Structures of isolated VapD further reveal how a symmetrical toxin homodimer adapts to interacting with an asymmetrical antitoxin and suggest how a primordial TA system evolved to become part of CRISPR-Cas immunity systems.
中文翻译:

VapXD 毒素-抗毒素系统中毒素抑制的结构基础
细菌 II 型毒素-抗毒素 (TA) 模块编码一种可下调代谢的有毒蛋白质和一种在正常生长过程中结合并抑制毒素的特定抗毒素。在不可分型流感嗜血杆菌(人类感染的常见原因)中,发现vapXD基因座构成功能性 TA 模块并有助于致病性;然而,VapD 的作用方式和 VapX 抗毒素的抑制机制仍然未知。在这里,我们报告了完整H的结构。流感VapXD 复合物,揭示了一种不寻常的 2:1 TA 分子化学计量,其中 VapD 的类 Cas2 同二聚体结合单个 VapX 抗毒素。VapX 由一个寡核苷酸/寡糖结合域组成,该域停靠在毒素二聚体上的不对称空腔中。分离的 VapD 的结构进一步揭示了对称毒素同源二聚体如何适应与不对称抗毒素的相互作用,并表明原始 TA 系统如何进化成为 CRISPR-Cas 免疫系统的一部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号