Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-10-22 , DOI: 10.1016/j.jmb.2020.10.020 Atanu Maiti 1 , Wazo Myint 1 , Krista A Delviks-Frankenberry 2 , Shurong Hou 3 , Tapan Kanai 4 , Vanivilasini Balachandran 1 , Christina Sierra Rodriguez 1 , Rashmi Tripathi 5 , Nese Kurt Yilmaz 3 , Vinay K Pathak 2 , Celia A Schiffer 3 , Hiroshi Matsuo 1
|
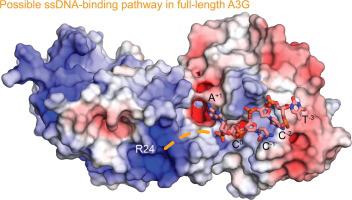
APOBEC3G (A3G) is a single-stranded DNA (ssDNA) cytosine deaminase that can restrict HIV-1 infection by mutating the viral genome. A3G consists of a non-catalytic N-terminal domain (NTD) and a catalytic C-terminal domain (CTD) connected by a short linker. While the CTD catalyzes cytosine deamination, the NTD is believed to provide additional affinity for ssDNA. Structures of both A3G domains have been solved individually; however, a full-length A3G structure has been challenging. Recently, crystal structures of full-length rhesus macaque A3G variants were solved which suggested dimerization mechanisms and RNA binding surfaces, whereas the dimerization appeared to compromise catalytic activity. We determined the crystal structure of a soluble variant of human A3G (sA3G) at 2.5 Å and from these data generated a model structure of wild-type A3G. This model demonstrated that the NTD was rotated 90° relative to the CTD along the major axis of the molecule, an orientation that forms a positively charged channel connected to the CTD catalytic site, consisting of NTD loop-1 and CTD loop-3. Structure-based mutations, in vitro deamination and DNA binding assays, and HIV-1 restriction assays identify R24, located in the NTD loop-1, as essential to a critical interaction with ssDNA. Furthermore, sA3G was shown to bind a deoxy-cytidine dinucleotide near the catalytic Zn2+, yet not in the catalytic position, where the interactions between deoxy-cytidines and CTD loop-1 and loop-7 residues were different from those formed with substrate. These new interactions suggest a mechanism explaining why A3G exhibits a 3′ to 5′ directional preference in processive deamination.
中文翻译:

可溶性 APOBEC3G 变体的晶体结构表明 ssDNA 在两个域之间延伸的通道中结合
APOBEC3G (A3G) 是一种单链 DNA (ssDNA) 胞嘧啶脱氨酶,可通过使病毒基因组发生突变来限制 HIV-1 感染。A3G 由一个非催化 N 端结构域 (NTD) 和一个催化 C 端结构域 (CTD) 组成,通过短接头连接。虽然 CTD 催化胞嘧啶脱氨基作用,但 NTD 被认为为 ssDNA 提供了额外的亲和力。两个 A3G 域的结构都已单独解决;然而,全长 A3G 结构一直具有挑战性。最近,全长恒河猴 A3G 变体的晶体结构得到解决,这表明二聚化机制和 RNA 结合表面,而二聚化似乎损害了催化活性。我们确定了 2.5 Å 的人类 A3G (sA3G) 可溶性变体的晶体结构,并从这些数据中生成了野生型 A3G 的模型结构。该模型表明 NTD 沿分子的主轴相对于 CTD 旋转了 90°,这种方向形成了一个连接到 CTD 催化位点的带正电荷的通道,由 NTD loop-1 和 CTD loop-3 组成。基于结构的突变,体外脱氨基和 DNA 结合分析以及 HIV-1 限制性分析确定位于 NTD 环 1 中的 R24 对与 ssDNA 的关键相互作用至关重要。此外,sA3G 显示在催化 Zn 2+附近结合脱氧胞苷二核苷酸,但不在催化位置,脱氧胞苷与 CTD 环 1 和环 7 残基之间的相互作用与底物形成的相互作用不同. 这些新的相互作用表明了一种机制,可以解释为什么 A3G 在进行性脱氨基中表现出 3' 到 5' 的方向偏好。


























 京公网安备 11010802027423号
京公网安备 11010802027423号