Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-10-29 , DOI: 10.1016/j.yjmcc.2020.10.012 Aleksei V Mikhailov 1 , Anuradha Kalyanasundaram 2 , Ning Li 2 , Shane S Scott 2 , Esthela J Artiga 2 , Megan M Subr 2 , Jichao Zhao 3 , Brian J Hansen 2 , John D Hummel 4 , Vadim V Fedorov 5
|
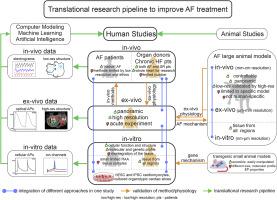
Atrial fibrillation (AF) occurrence and maintenance is associated with progressive remodeling of electrophysiological (repolarization and conduction) and 3D structural (fibrosis, fiber orientations, and wall thickness) features of the human atria. Significant diversity in AF etiology leads to heterogeneous arrhythmogenic electrophysiological and structural substrates within the 3D structure of the human atria. Since current clinical methods have yet to fully resolve the patient-specific arrhythmogenic substrates, mechanism-based AF treatments remain underdeveloped. Here, we review current knowledge from in-vivo, ex-vivo, and in-vitro human heart studies, and discuss how these studies may provide new insights on the synergy of atrial electrophysiological and 3D structural features in AF maintenance. In-vitro studies on surgically acquired human atrial samples provide a great opportunity to study a wide spectrum of AF pathology, including functional changes in single-cell action potentials, ion channels, and gene/protein expression. However, limited size of the samples prevents evaluation of heterogeneous AF substrates and reentrant mechanisms. In contrast, coronary-perfused ex-vivo human hearts can be studied with state-of-the-art functional and structural technologies, such as high-resolution near-infrared optical mapping and contrast-enhanced MRI. These imaging modalities can resolve atrial arrhythmogenic substrates and their role in reentrant mechanisms maintaining AF and validate clinical approaches. Nonetheless, longitudinal studies are not feasible in explanted human hearts. As no approach is perfect, we suggest that combining the strengths of direct human atrial studies with high fidelity approaches available in the laboratory and in realistic patient-specific computer models would elucidate deeper knowledge of AF mechanisms. We propose that a comprehensive translational pipeline from ex-vivo human heart studies to longitudinal clinically relevant AF animal studies and finally to clinical trials is necessary to identify patient-specific arrhythmogenic substrates and develop novel AF treatments.
中文翻译:

综合评价人心房心肌的电生理和 3D 结构特征,了解心房颤动维持机制
心房颤动 (AF) 的发生和维持与人心房的电生理学(复极和传导)和 3D 结构(纤维化、纤维取向和壁厚)特征的进行性重塑有关。AF 病因的显着多样性导致人类心房 3D 结构内的异质致心律失常电生理和结构底物。由于目前的临床方法尚未完全解决患者特异性致心律失常的底物,因此基于机制的 AF 治疗仍然不发达。在这里,我们回顾了来自体内、离体和体外人类心脏研究的当前知识,并讨论这些研究如何为心房电生理学和 3D 结构特征在 AF 维持中的协同作用提供新的见解。对通过手术获得的人体心房样本进行的体外研究为研究广泛的 AF 病理学提供了一个很好的机会,包括单细胞动作电位、离子通道和基因/蛋白质表达的功能变化。然而,样本的有限大小阻碍了对异质 AF 基板和重入机制的评估。相比之下,冠状动脉灌注的离体人类心脏可以通过最先进的功能和结构技术进行研究,例如高分辨率近红外光学映射和对比增强 MRI。这些成像方式可以解决房性心律失常底物及其在维持 AF 的折返机制中的作用,并验证临床方法。尽管如此,纵向研究在移植的人类心脏中是不可行的。因为没有完美的方法,我们建议将直接人体心房研究的优势与实验室中可用的高保真方法和现实的患者特定计算机模型相结合,将阐明对 AF 机制的更深入了解。我们建议,从体外人类心脏研究到纵向临床相关 AF 动物研究,最后到临床试验的综合转化管道对于确定患者特异性致心律失常底物和开发新的 AF 治疗是必要的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号