Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-10-24 , DOI: 10.1016/j.yjmcc.2020.10.006 Maike Schuldt 1 , Jamie R Johnston 2 , Huan He 3 , Roy Huurman 4 , Jiayi Pei 5 , Magdalena Harakalova 5 , Corrado Poggesi 6 , Michelle Michels 4 , Diederik W D Kuster 1 , Jose R Pinto 2 , Jolanda van der Velden 1
|
Background
The clinical outcome of hypertrophic cardiomyopathy patients is not only determined by the disease-causing mutation but influenced by a variety of disease modifiers. Here, we defined the role of the mutation location and the mutant protein dose of the troponin T mutations I79N, R94C and R278C.
Methods and results
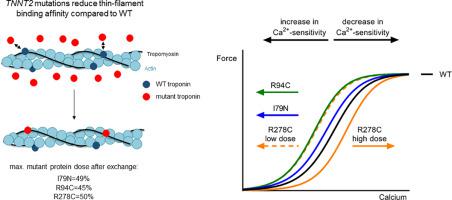
We determined myofilament function after troponin exchange in permeabilized single human cardiomyocytes as well as in cardiac patient samples harboring the R278C mutation. Notably, we found that a small dose of mutant protein is sufficient for the maximal effect on myofilament Ca2+-sensitivity for the I79N and R94C mutation while the mutation location determines the magnitude of this effect. While incorporation of I79N and R94C increased myofilament Ca2+-sensitivity, incorporation of R278C increased Ca2+-sensitivity at low and intermediate dose, while it decreased Ca2+-sensitivity at high dose. All three cTnT mutants showed reduced thin filament binding affinity, which coincided with a relatively low maximal exchange (50.5 ± 5.2%) of mutant troponin complex in cardiomyocytes. In accordance, 32.2 ± 4.0% mutant R278C was found in two patient samples which showed 50.0 ± 3.7% mutant mRNA. In accordance with studies that showed clinical variability in patients with the exact same mutation, we observed variability on the functional single cell level in patients with the R278C mutation. These differences in myofilament properties could not be explained by differences in the amount of mutant protein.
Conclusions
Using troponin exchange in single human cardiomyocytes, we show that TNNT2 mutation-induced changes in myofilament Ca2+-sensitivity depend on mutation location, while all mutants show reduced thin filament binding affinity. The specific mutation-effect observed for R278C could not be translated to myofilament function of cardiomyocytes from patients, and is most likely explained by other (post)-translational troponin modifications. Overall, our studies illustrate that mutation location underlies variability in myofilament Ca2+-sensitivity, while only the R278C mutation shows a highly dose-dependent effect on myofilament function.
中文翻译:

引起 HCM 的肌钙蛋白 T 突变的突变位置决定了人类心肌细胞肌丝功能障碍的程度
背景
肥厚型心肌病患者的临床结果不仅由致病突变决定,还受到多种疾病修饰因素的影响。在这里,我们定义了肌钙蛋白T突变I79N、R94C和R278C的突变位置和突变蛋白剂量的作用。
方法和结果
我们测定了透化的单个人类心肌细胞以及含有 R278C 突变的心脏病患者样本中肌钙蛋白交换后的肌丝功能。值得注意的是,我们发现小剂量的突变蛋白足以对 I79N 和 R94C 突变的肌丝 Ca 2+敏感性产生最大影响,而突变位置决定了这种影响的大小。I79N和R94C的掺入增加了肌丝Ca 2+敏感性,而R278C的掺入增加了低剂量和中剂量时的Ca 2+敏感性,而降低了高剂量时的Ca 2+敏感性。所有三种 cTnT 突变体均表现出细丝结合亲和力降低,这与心肌细胞中突变肌钙蛋白复合物相对较低的最大交换(50.5 ± 5.2%)一致。相应地,在两个患者样本中发现了 32.2 ± 4.0% 突变 R278C,其显示 50.0 ± 3.7% 突变 mRNA。根据显示具有完全相同突变的患者的临床变异性的研究,我们观察到具有 R278C 突变的患者的功能性单细胞水平的变异性。肌丝特性的这些差异不能用突变蛋白数量的差异来解释。
结论
利用单个人心肌细胞中的肌钙蛋白交换,我们发现TNNT2突变诱导的肌丝 Ca 2+敏感性变化取决于突变位置,而所有突变体均表现出细丝结合亲和力降低。观察到的 R278C 特异性突变效应无法转化为患者心肌细胞的肌丝功能,很可能是通过其他(后)翻译肌钙蛋白修饰来解释的。总体而言,我们的研究表明,突变位置是肌丝 Ca 2+敏感性变异的基础,而只有 R278C 突变对肌丝功能显示出高度剂量依赖性的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号